Question #1:
Consider the two beakers containing water, both at the same initial temperature,
say 25 degrees Celsius.
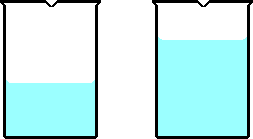
The beaker on the left has 25 mLs of water and the beaker on
the right has 50 mLs of water. Both have the same initial temperature. If I
add the same amount of heat to both beakers, using a bunsen burner, which has
the higher temperature?
Answer:
The beaker on the left, containing the smaller amount of water,
will have the higher temperature. When the same amount of heat is added to both
beakers the concept of specific heat says the smaller the amount of substance
the larger the change in temperature.
At anytime use the back button in the Navigation Toolbar to return
to the PLE2 page.