Question #3:
Consider the two beakers containing water, both at the same initial temperature,
say 25 degrees Celsius.
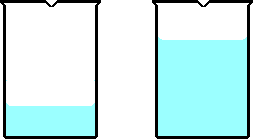
If twice as much heat is added to the beaker on the right compared
to the beaker on the left, and the final temperature of the beaker on the right
is lower than the beaker on the left, which beaker has more water?
Answer:
The beaker on the right must have a more water compared to the
beaker on the left. If more heat is added to the beaker on the left compared
to the beaker on theright there must be more water for the temperature change
to be smaller.
Specific heat is a measure of the amount of heat required to
change the temperature of 1 gram of a substance by one degree Celsius. Specific
heat has units of J g-1 C-1.
For water the specific heat is 4.184 J g-1 C-1.
Use the back button in the Navigation Toolbar to return to the
PLE2 page.