![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 8: MOLECULAR WEIGHT OF A
VOLATILE LIQUID
![]()
The variable n can be written as

Substitute this quotient for n in the ideal gas equation and solve for the molar mass.
![]()
![]()
EXPERIMENT 8: MOLECULAR WEIGHT OF A VOLATILE LIQUID
INTRODUCTION: Determination of the Molecular Weight of a Volatile Liquid by the Dumas Method
The Dumas method is one of the simplest ways to measure the molecular weight of a substance. It uses the ideal gas law. In this method we have to confine a sample of gas in a container of known volume, making sure when we do this that the sample is exactly at atmospheric pressure. The temperature and the mass of the gas sample have to be measured, along with the atmospheric pressure. Because liquids are much easier to handle than gases, a volatile liquid is usually used as the source of the gas. The liquid must have a boiling point substantially above room temperature and below the boiling point of water in order for this method to work well. The paragraphs below describe the experimental method generally used by scientists and the modifications to that method that we will make in our experiment. As you read, see if you can identify the possible sources of error that our simplified procedure will introduce. In the classic experiment, a round thin walled glass bulb with a long, thin bent neck is made by a glassblower and weighed. A small amount of volatile liquid is introduced into the bulb through a tiny opening at the end of the bulb's neck. The bulb is heated in a boiling water bath to vaporize the liquid. The volume of vapor formed at 100 ºC (the approximate temperature of the boiling water - the exact temperature of the boiling water must be measured experimentally) and atmospheric pressure is greater than the volume of the bulb. The vapor first pushes all of the air out of the bulb and then begins to rush out of the opening until the pressure inside the bulb equals the pressure outside (atmospheric). With careful observation, the vapor can be seen exiting the bulb. It looks like a jet engine exhaust or a swirling cloud. When all of the liquid is vaporized and no more vapor is seen leaving the bulb, the bulb contains a sample of vapor at atmospheric pressure and 100 ºC with a volume exactly equal to the volume of the bulb. The opening in the neck of the bulb is quickly sealed with a flame. It is important that the bulb be sealed at exactly the moment that vapor stops escaping from the bulb. (Sealing before vapor stops escaping will result too much vapor remains in the bulb. The molecular mass calculated will be too high. Waiting too long to seal the opening will result in a molecular weight that is too low. Can you explain why?) The sealed bulb is cooled, dried and carefully reweighed. The volume of the bulb must now be determined. The vapor inside has condensed, and the space above the liquid in the bulb is a near vacuum. The sealed tip of the bulb is then cut off with a file under water in a large container . The water rushes into the bulb, filling it completely. The bulb is dried on the outside and weighed once again. The volume of the bulb can be determined from the mass of water it contains. Barometric pressure and room temperature are carefully measured and recorded. The density of water at room temperature is either measured or obtained from the chemical literature. The volume (V) of the bulb is calculated from the mass of the water needed to completely fill the bulb. The mass (g) of vapor is obtained by subtraction. The temperature of the boiling water bath (T) and atmospheric pressure (P) were measured directly during the experiment. With this information, the molecular mass of the gas can be calculated using the ideal gas law. We will make a few simplifications in our experiment. First, we will use an ordinary Erlenmeyer flask covered with aluminum foil rather than a glass bulb. A tiny hole will be made in the foil to allow vapor to escape from the flask. We will not seal the flask when vapor stops escaping from the hole. We will simply remove it from the hot water bath as quickly as possible. The vapor will condense rapidly. We will measure the volume of the flask by filling it with water and then measuring the volume of water using a graduated cylinder. The information needed to determine the molecular mass of the unknown is the same as in the classic Dumas method: pressure (P), volume ,(V) the mass of the vapor,(g), and the temperature (T). Using this procedure, we should be able to determine the molecular mass of a volatile liquid to within 10% error.
![]()
![]()
EXPERIMENT 8: MOLECULAR WEIGHT OF A
VOLATILE LIQUID
![]() Top
Top
| EQUIPMENT: | |
| aluminum foil ......................3 inch square | matches or lighter ....................... several |
| balance.....................................1 | paper towels ............................. several |
| barometer..................................1 | ring stand ....................................2 |
| beaker, 50 mL ............................1 | rubber band..................................1 |
| beaker, 600 mL...........................1 | rubber tubing ................................1 |
| Bunsen burner ............................1 | small pin .....................................1 |
| flask, Erlenmeyer, 125 mL........... 1 or 2 | thermometer, ºC.............................1 |
| graduated cylinder, 100 mL or larger ..1 | utility clamp..................................1 |
| graduated cylinder, 10 mL ..............1 | wire gauze ...................................1 |
| iron ring clamp............................1 |
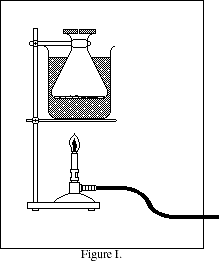 Attach a utility clamp to a 125 mL
Erlenmeyer flask. Immerse it as deeply
as possible into a 600 mL beaker. Pour
water into the beaker around the outside
of the flask until the beaker is almost full
- allow room for vigorous boiling. It is
important that no water get inside the
flask! Remove the flask and add a few
boiling chips to the water in the beaker.
Heat the water to boiling over a Bunsen
burner.
While the water is heating, remove
the clamp from the flask and dry the out
side of the flask thoroughly with a paper
towel. Place a small square of aluminum
foil over the mouth of the flask . Loosely
fold the foil down around the flask's
neck. Secure the foil in place with a
rubber band. Make a small hole in the
center of the foil with a straight pin. Be
certain that the hole is open and not
partially blocked by displaced aluminum
foil. Weigh the flask, foil cap, and
rubber band to the nearest milligram and
record the mass in Obs #1.
Attach a utility clamp to a 125 mL
Erlenmeyer flask. Immerse it as deeply
as possible into a 600 mL beaker. Pour
water into the beaker around the outside
of the flask until the beaker is almost full
- allow room for vigorous boiling. It is
important that no water get inside the
flask! Remove the flask and add a few
boiling chips to the water in the beaker.
Heat the water to boiling over a Bunsen
burner.
While the water is heating, remove
the clamp from the flask and dry the out
side of the flask thoroughly with a paper
towel. Place a small square of aluminum
foil over the mouth of the flask . Loosely
fold the foil down around the flask's
neck. Secure the foil in place with a
rubber band. Make a small hole in the
center of the foil with a straight pin. Be
certain that the hole is open and not
partially blocked by displaced aluminum
foil. Weigh the flask, foil cap, and
rubber band to the nearest milligram and
record the mass in Obs #1.
![]()
![]()
Obtain a small sample of an unknown liquid. Record its number and physical description as Obs. #2.

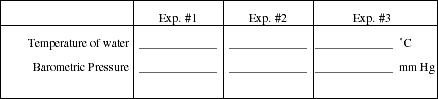
Remove the foil cap and add about 2 mL of unknown liquid to the flask. Replace the foil and rubber band. Clamp the flask to the ring stand and immerse the flask as deeply as possible in the boiling water. Measure the temperature of the boiling water and record the value in Obs. #3. Also read the barometer and record the air pressure. Watch the liquid in the flask. As it begins to boil, follow the vapor up through the flask with your eye. Soon vapor will start to escape through the pin hole. The vapor will be easy to see if you are at eye level with the top of the flask and look across the top of the flask. It also helps to look toward a light source, such as a window. The vapor will look like a colorless swirling cloud or jet. When all of the liquid has vaporized, including any droplets that may have formed on the neck, no more vapor is seen escaping through the pin hole (this should take no more than 1 to 2 minutes). When the vapor stops escaping, very quickly remove the flask and set it on a folded paper towel. Remove the clamp from the neck of the flask. Dry the outside of the flask carefully with a paper towel. Be sure to dry under and around the folds of aluminum foil. Allow the flask to come to room temperature. As the flask cools, you will see a small amount of liquid condense in the bottom of the flask.
![]()
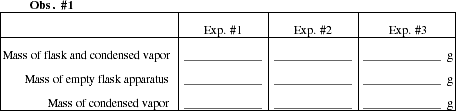
When the flask has returned to room temperature, weigh the flask and contents and record
the mass in Obs. #1. Determine the mass of the condensed vapor.
If you have time to collect an additional set of data, remove the foil cap from the flask and
add 1 to 2 mL of your unknown. Replace the cap and heat the flask as described above. After
heating and cooling the flask, reweigh and record the mass of the flask and contents in Obs. #1.
![]()
![]()
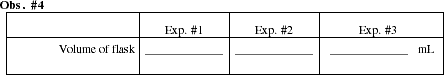
After the final weighing, remove the foil and rinse out the flask with water. Completely fill the flask, level full, with water. Measure the volume of the flask by measuring the volume of water that it can hold. Pour the water in the flask into a graduated cylinder. You may have to fill the cylinder several times to accommodate the volume of water in the flask. Record the total volume of the flask in Obs. #4.
Space has been provided for three experimental runs. The number of runs you complete will depend on the amount of time available. Ask your instructor how many runs you are expected to complete.
![]()
![]()

![]()
![]()
% error =
![]() x 100
x 100
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments