![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 7: CHARLES' LAW
![]()
![]()
![]()
![]()
EXPERIMENT 7: CHARLES' LAW
![]() Top
Top
| EQUIPMENT: | |
| beaker, 50 mL.........................1 | Charles' Law tube......................1 |
| beaker, 600 mL .......................1 | 6" ruler, graduated in mm .............1 |
| boiling chips........................several | rubber bands ............................2 |
| Bunsen burner.........................1 | 1 hole, slit rubber stopper or cork....1 |
| rubber tubing.....................18" | utility clamp .............................1 |
| matches .........................several | thermometer, ºC ........................1 |
| ring................................. 1 | pan .......................................1 |
| ring stand..........................1 | paper towels......................... several |
| wire gauze.........................1 | spatula ...................................1 |
| glass rod ...............................1 |
![]()
Procedure:
WARNING: In this experiment you will be working with a glass tube containing mercury. Mercury is an extremely toxic substance. As a precaution, remove any gold jewelry before you begin this experiment. If any mercury should escape from the tube, note the resting place of the tiny droplets formed, and call your instructor at once. Do not allow any mercury to touch your skin.
Pour about 500 mL of water into a 600 mL beaker. Add 1 or 2 boiling chips. Heat the water over a Bunsen burner until the water boils.
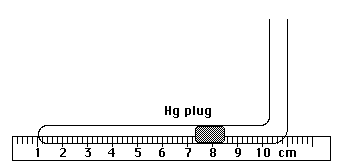
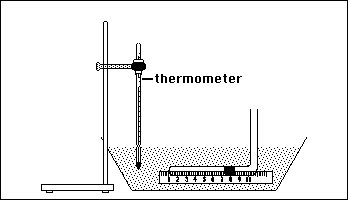
While the water is heating, obtain a Charles' Law tube from your instructor. Handle the tube very gently. Dropping or shaking the tube can cause the mercury plug to shift or separate, making the tube unusable. Place the assembled apparatus in a shallow pan. The figure below shows the equipment setup you will use in this experiment.
Wet the inside of the hole in the rubber stopper and the thermometer bulb to be inserted with a glycerine/water solution. Wrap the top two-thirds of the thermometer with a piece of paper towel and carefully insert the bulb into the stopper with a slight rotating movement. Ease the thermometer through the split hole (DO NOT FORCE) of the stopper.
![]()
![]()
Poor technique can result in serious cuts or punctures. If you are unsure how to proceed ask your instructor. When the water begins to boil, turn off the heat. Pour the water into the pan. (Note: If it is very cold in the laboratory, warm up the pan by first rinsing it with a few mL of hot water.) Enough water should be in the pan to submerge the horizontal portion of the ruler/Charles Law tube apparatus, yet leave a portion of the end beyond the bend above the water surface. If the horizontal portion of the glass tube is not completely submerged, add some tap water. Support the thermometer using a utility clamp attached to a ring stand. The thermometer bulb should be completely submerged, but should not touch the walls or the bottom of the pan. Have your instructor check your equipment.
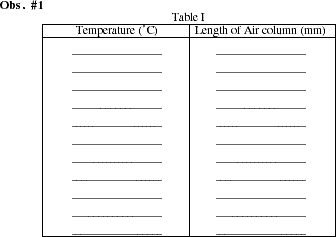
Simultaneously read the temperature and the length of the air column in the tube. Record these values in Table I. Record temperature and column length at approximately 5 to 10 ºC intervals as the temperature falls. The length of the air column should change by 1 millimeter or more between subsequent readings. Occasionally stir the water using a stirring rod to prevent temperature gradients from forming in the water. If the length of the air column does not change by at least 1 mm with a 10 ºC temperature interval, consult your teacher. Begin adding small amounts of ice to the water following the second or third temperature reading. Stir the water with a glass rod to maintain a constant temperature throughout the bath. Continue taking readings at 5 to 10 ºC intervals. When the temperature falls below room temperature (about 20 ºC) larger amounts of ice will have to be added to maintain a falling temperature. Continue to take readings until a temperature near 0 ºC is obtained. Near 0 ºC, continue to add ice, but also add some rock salt. Stir the mixture until a constant temperature (below 0 ºC) is obtained. Record a final measurement.
Remove the apparatus from the water bath. Carefully wash and dry the outside of the Charles' Law tube and return it to your instructor. Clean the thermometer before putting it away. Be sure to clean and dry the pan. (Metal pans may rust rapidly if salt water is left on them.)
![]()
![]()
![]()
![]()
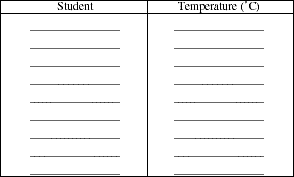
Determine the average of the value for absolute zero in your class.
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments