![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 2: Rutherford's Model of the Atom
Obtain copies of the two Rutherford computer disks+, labeled Rutherford #1 (Introduction and Explanation)) and Rutherford #2 (Experiment Simulation Tool). Place Rutherford #1 into disk drive #1 and turn the computer on. The disk will "autoboot". The main menu is displayed with three choices;
![]()
Part 1 - Introduction and Explanation
From the main menu, select option 1, "Introduction and Explanation", by typing 1 followed by RETURN. A new menu will appear;

For instructions on using the computer, type 1 and then RETURN. A single screen containing input instructions will be displayed. Read the information and then press RETURN to go back to the menu. To review the background information on the experiment, select option 2. To move to the next screen press the 'RETURN' key. Some of the screens will not allow you to continue until the animation is complete. The message, 'RETURN' to continue, 'ESC' for menu, will appear when you can continue. When you complete the review covering the background of the experiment, the program will automatically proceed to the explanation of Rutherford's theory. Read the information contained in the background and explanation sections carefully and answer the questions listed below.
Answer the following questions during your review of the background and explanation sections:
1. Draw the apparatus of Rutherford's experiment and identify each component.
![]()
![]()
+ The 2-disk program was written by Robert C. Rittenhouse, Department of Chemistry, Walla Walla College,
College Place, WA 99324. Available from Project SERAPHIM, Department of Chemistry, University of
![]()
![]()
2. Briefly describe the basic experiment performed by Rutherford.
![]()
3. What unexpected occurrence happened during the experiment? How did Rutherford
explain this?
![]()
4. What equation did Rutherford formulate to predict the relative number of particles
scattered at any angle? Define all variables.
![]()
The last screen in the section explaining the theory behind Rutherford's experiment
provides a different option for continuing. If you press "RETURN' you will have to
remove Rutherford disk #1 and insert Rutherford disk #2 to continue the experiment. If
you select 'ESC' you will return to the main menu and you must select option 2 to continue
the experiment.
![]()
![]()
EXPERIMENT 2: Rutherford's Model of the Atom
![]() Top
Top
Part 2 - Experiment Simulation Tool
Set Up:
After inserting Rutherford disk #2 and pressing RETURN, a short introduction will appear on the screen that briefly describes two types of experiments . Read the information and then press RETURN to continue. The main menu will appear on the screen:
![]()

Select option 1, "List and Description of Simulated Experimental Tools". Read the information and then press RETURN to return to the main menu. For future reference, the list of simulated tools is given below:

Then from the main menu, select option 2, "Experiment Design and Implementation", to begin the experiment. A new menu will appear to choose the level of experimentation:
![]()
Select option 1, "Macroscopic", from this menu.
![]()
![]()
Macroscopic
For this experiment, the number of alpha particles at different scattering angles will be measured. After selecting the macroscopic portion of this experiment, a list of variables will be displayed.
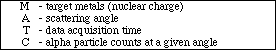
The computer can generate a data file in which data for the two variables selected can be stored. You will not be using these data files. Instead, you will record your data in the tables provided. However, you must choose two variables to record in order to continue with the experiment. Type "A" (for scattering angle) after the computer asks for the x- variable, and type "C" (for alpha particle counts at a given angle) after it asks for the y- variable. A graph with a list of options will then appear on the screen. These options can be selected by pressing the letter that is in brackets (for example; to select <M>, type "M").
Before you begin the experiment, the metal target, the timer, and the scattering angle must be set. Select M to specify the metal for the target foil. A list of target foils and their atomic numbers are given. Select gold by entering "AU" followed by a RETURN. The program will then return to the graph and the list of options.
Enter the time in minutes for the experiment to run by selecting "T" and then typing "5" followed by RETURN. This will simulate the collection of alpha particle scattering data for a period of 5 minutes. The experiment will not actually last 5 minutes but only a few seconds.
Select "A" for the scattering angle and enter "2" followed by RETURN. This tells the computer to display the number of alpha particles with a scattering angle of 2º.
You are now ready to start the experiment .
Type "S" to begin collecting the data for the number of alpha particles. The computer will then begin plotting the number of alpha counts versus the scattering angle. After the computer has completed recording the data, it will display the number of alpha particle counts with a scattering angle of 2º in the lower right hand corner labeled COUNTS. Record the data in the Table 1. Continue this experiment by obtaining the number of alpha particles scattered at different angles with gold foil as the target metal. This can be done by selecting "A" and entering the new scattering angle. The computer will then display the new alpha particle count in the box labeled COUNTS. Change the scattering angle to the following by using the procedure described above: 3º, 4º, 6º, 8º, 10º, 15º, 20º, 40º,and 60º. Record the number of alpha-particle counts in the data table for each scattering angle.
The second part of the macroscopic experiment involves collecting data for a different target foil with all the other variables remaining constant. Select "R" to reset the graph and the variables. Then, select "M" to select a copper metal foil target. Reset the timer to 5 minutes and the scattering angle to 2º as described in the gold foil experiment. Start the experiment by selecting "S". Record your data in the table below. Next, change the scattering angle to 3º, 4º, 6º, 8º, 10º, 15º, 20º, 40º and 60º. Record the alpha particle counts for each angle. After obtaining all the necessary data, select "Q" to quit this part of the experiment and to go back to the Main Menu.
![]()
![]()
TABLE 1:
Questions
5. Compare the alpha-particle counts for the gold foil for the different angles. Are any trends evident in the values? If so, how can these trends be explained?
![]()
6. Compare the data obtained in the gold foil experiment to the data from the copper foil
experiment. Discuss the reasons for any similarities or differences you observe.
![]()
7. (a) Calculate the fraction of alpha particles scattered by the gold foil at 4º and 20º
using Rutherford's formula (written in question 4).
![]()
![]()
(b) Calculate the fraction of alpha particles scattered by the copper foil at 4º and 20º.
![]()
(c) How do these calculations support the data in Table I? (Keep in mind you
calculated fractions and recorded actual counts.)
![]()
![]()
Microscopic
Select 2, "Experiment Design and Implementation", from the Main Menu. Again, the menu to choose the level of experimentation will appear on the screen. Select 2, "Microscopic". In this experiment you will determine the relationship between kinetic energy of the incident particle and the particle's scattering angle. A list of variables that can be used to generate a data file will appear.

Although you will be recording your data in the tables provided, the computer will not continue unless you enter two variables. Enter "E" for the x-variable and "A" for the y- variable.
A screen containing information on the experiment will appear. Read this information. At the bottom of this screen, the computer will prompt you to enter the magnification power of the digital microscope.
Enter "250000000" (Note: seven zeros and no commas!) for the magnification power. An atom, an impact parameter scale, and several options will then appear on the screen.
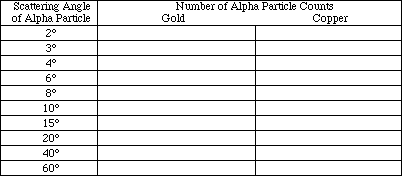
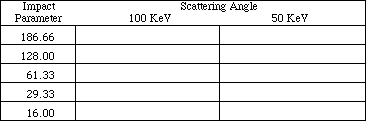
On the right of the screen is a list of the different variables and their values. Check to make sure that the target metal is gold, Au, and that the energy is 100 KeV. (If not, select the appropriate options and set them to the above information). The other variable that needs to be set before beginning this experiment is the impact parameter. Set the impact parameter to 186.66 picometers by selecting "I" and using the arrow keys to increase or decrease the parameter that is listed under the heading IMP.PAR.(pm). When the parameter is set, press "E" to exit.
You are now ready to begin.
Select "A" to start the experiment. The diagram will graph the path of the particle that has the given energy and impact parameter. The computer will display the scattering angle in the lower right hand corner under the heading ANGLE (deg.). Record the impact parameter and the angle in Table 2. Change the impact parameter to 128, then to 61.33, 29.33, and finally 16 by selecting "I" and using the arrow keys. Remember to press "A" after each new setting to obtain the data. Record the data for each impact parameter in Table 2. When the parameter is set, press "E" to exit.
To complete Table 2, set up a similar experiment with all the variables remaining the same except the energy of the alpha particle.
Select "R" to reset the graph and the variables. The screen containing the introductory information and magnification power will appear.
![]()
![]()
Enter "250000000" for the microscope's magnification power. The atom, impact parameter scale, and several options will appear again. The metal should still be set for gold, "AU".
Change the kinetic energy to 50 KeV by selecting "E" and entering "50" followed by a RETURN.
Change the impact parameter successively to 186.66, 128, 61.33, 29.33 and finally to 16 as instructed before, and press "A" after each new setting. Record the data in Table 2.
TABLE 2:
METAL: Gold
Questions:
8. At a given particle energy, how does the impact parameter affect the scattering angle? Discuss the theory that explains this relationship.
![]()
9. Compare the scattering angles at the two energies recorded in Table 2. Is there a
relationship between the energy of the particles and their scattering angles?
![]()
![]()
Student Experiment
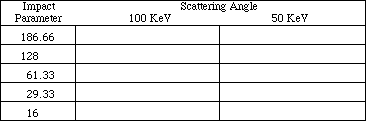
In this section you are to repeat the experiment just performed using a different metal target foil of your choice. Reset the graph and use 250000000 for magnification in order to be able to accurately compare results of your new experiment with those obtained previously.
TABLE 3:
METAL: ______________
![]()
Questions
![]()
10. From your data, what conclusions can you make about the effect the atomic size has
on the scattering angle?
![]()
After collecting your data, select "Q" to quit the experiment and go back to the Main Menu.
From the Main Menu select option 6 ,QUIT, and press RETURN to quit the program. Take the
Rutherford Disk #2 out of the disk drive and turn the computer off.
![]()
![]()
Determining Nuclear Radius (optional)
Rutherford concluded from his experiments, that almost all of the mass of an atom was concentrated into a tiny "nucleus" at the atom's center. We would like to be able to measure the radius of the nucleus, but because it is so tiny, it is difficult to measure directly. Instead, we will attempt to estimate the radius based on a few experimental observations.
We have observed that alpha particles which pass very near the nucleus are deflected back through large angles. Suppose we could fire an alpha particle directly at a gold nucleus (with zero impact parameter) so that a "head-on" collision would occur. The gold nucleus is much larger and heavier than the alpha particle (a helium nucleus). We would expect that the alpha particle would smash into the gold nucleus and bounce straight back (at a scattering angle of 180º) like a rubber ball bouncing off a brick wall. However, the situation is not quite so simple. Unlike the rubber ball and the brick wall, the alpha particle and the gold nucleus each have a positive charge. Since like charges repel, the alpha article never actually touches the gold nucleus. How close the alpha particle gets to the nucleus depends on its initial kinetic energy. The faster the alpha particle is moving the more energy it has, and the closer it can get to the nucleus before being pushed away. When the alpha particle reaches this closest approach distance from the nucleus, its kinetic energy is exactly equal to the repulsive electrostatic potential of the two positive charges. The alpha particle stops for an instant and then is pushed back, away from the nucleus.
The general equation for the electrostatic potential energy between two charged bodies follows.

Electrostatic potential energy between two specific nuclei can be calculated by substituting for Q in equation (1) above. For a specific nucleus,

Equation (2) is the electrostatic potential energy between two charged nuclei. Recall from above that when the alpha particle reaches its closest approach distance from the nucleus, its kinetic energy is exactly equal to the repulsive electrostatic potential of the two
![]()
![]()
positive charges. At the closest approach the kinetic energy of the alpha particle is given by equation (2).
![]()
If equation (2) is set equal to the initial kinetic energy of the incoming alpha particle,
then Z1, the atomic number of the alpha particle, can be set to 2. The distance, r, becomes
ro, the closest distance that the alpha particle can get to the nucleus.
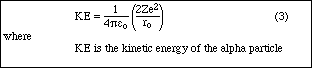
Solving the equation for ro gives:
![]()
![]()
Equation (4) allows the determination of the closest approach of an alpha particle to
a nucleus of known atomic number. The distance, ro, will always be larger than the radius
of the nucleus. If we want to make ro approach the actual radius of the nucleus, we must
use the most energetic alpha particle we can find. The highest kinetic energy alpha
particles found in nature have 7.7 MeV (1.2 x 10-12 J) of energy. Particle accelerators have
been used to generate higher energy alpha particles.
11. How close to the nucleus could an alpha particle with 7.7 MeV kinetic energy get to the nucleus of a gold atom?
![]()
12. Estimate the radius of a gold nucleus.
![]()
![]()
13. The radius of a gold atom is about 1.4 x 10 -10 m. What percent of the volume of a gold atom is occupied by the nucleus?
![]()
![]()
14. Repeat question #11 for a copper atom.
![]()
15. Compare the sizes of the gold and copper atom nuclei. Do these comparisons
correspond to the data collected in the macroscopic section of this experiment?
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments
![]()