![]() Pre-lab Questions
Pre-lab Questions
![]() Experiment
Experiment
![]() Post-lab Questions
Post-lab Questions
EXPERIMENT 16: CATION ANALYSIS
The following preparatory questions should be answered before coming to class. They are intended to introduce you to several ideas important to aspects of the experiment. You must turn- in your work to your instructor before you will be allowed to begin the experiment. Be sure to bring a calculator and paper to laboratory.
1. Read the sections in your text concerning solubility rules.
![]()
2. Using the solubility information in your text, complete the following table. (Simply enter
soluble (S) or insoluble (I) in each compartment.)
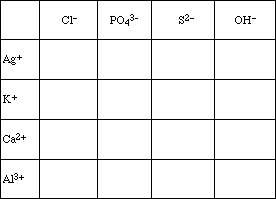
![]()
3. Design a scheme to separate a mixture of the four cations listed in question 2.
![]()
![]()
EXPERIMENT 16: CATION ANALYSIS
![]() Top
Top
EQUIPMENT:

INTRODUCTION:
It is VERY important that you take care to prevent contamination because of the large number of experiments that will be performed in this experiment. Be sure to use deionized water in all chemical tests. Even the smallest amount of contamination can cause changes the results of your tests. This will lead you to record incorrect observations and to make wrong conclusions. As you are performing these experiments individually, you will have no one but yourself to blame for such errors. Following every test you should carefully clean the equipment with deionized water. Cleaning your test tubes and droppers following an experiment can prevent contamination on a subsequent experiment.
You should take care to label the vials which contain important stock solutions. Labels should be available in the laboratory. When testing how the cations behave to a specific reagent, perform tests on all ions simultaneously. Be sure that test tubes and/or the locations of the test tubes in the test tube rack are clearly labeled. Record observations immediately after each test. Following the test of all anions, check each test tube a second time to strengthen your observations.
When writing your observations be sure to note colors of precipitates and supernatant solutions and precipitate texture. Are gases formed? Are reactions exothermic, endothermic, or is it difficult to tell? Remember, 'no reaction' is an important observation. Some combinations of reagents do not react.
All observed reactions must include balanced chemical equations. Your instructor can help - so ask.
![]()
![]()
+ This experiment is a modified version of an experiment written by Michael R. Abraham and Michael J.
Pavelich from Inquiries Into Chemistry published by Waveland Press, Inc.
![]()
![]()
Part I: Qualitative Analysis of Cations
![]()
Obtain 1 mL samples of 0.5 M solutions of the salts AgNO3, Pb(NO3)2, Cu(NO3)2,
Ba(NO3)2, and Fe(NO3)3 in separate clean, labeled vials or test tubes. Dilute each solution to a
final volume of 5 mL using deionized water. These will be your 'Test Solutions'. Identify the
formulas and charges for the cations on the line below. Have your instructor check them before
continuing.
![]()
Each of the cation solutions should be subjected to each of the following tests. Record the cation and your observations following each experiment.
Chemical Tests: All tests should be performed in clean test tubes sized to fit the centrifuge.
Test #1
![]()
To 20 drops of each test solution add 7 drops of 3 M H2SO4.
![]()
Obs. #1
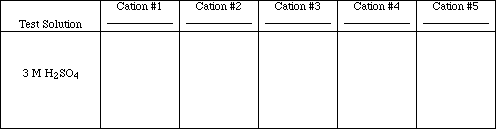
Write the ionic and net ionic equations for those tests which resulted in chemical reactions.
Rxn. #1
![]()
Which of the cations could be separated from a solution containing all five using Test #1?
Could any of the ions be identified using only test #1?
Expl. #1
![]()
![]()
Test #2
Add 20 drops of each test solution to a clean 13 x 100 test tube. Add 7 drops of 6 M HCl each test tube. Any precipitates that are formed should be centrifuged and washed twice with deionized water. Decant the supernatant fluid. Save the precipitates for test #3.
Obs. #2
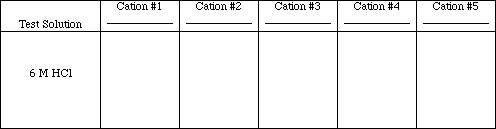
Write the ionic and net ionic equations for those tests which resulted in chemical reactions.
Rxn. #2
![]()
Test #3
![]()
Add 30 drops of deionized water to each precipitate formed in Test #2 (Note: If no
precipitate formed in Test #2 for a particular cation, place an X in the box for that ion, no test need
be performed.). Stir the precipitate and water to mix thoroughly. Heat the sample in a boiling
water bath for approximately 4 minutes, stirring occasionally. Centrifuge the test tubes, then
decant the hot liquid and add 1 drop of 1.0 M potassium chromate (K2CrO4) solution to each.
![]()
Obs. #3
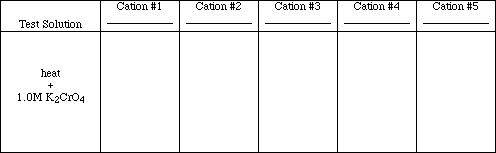
![]()
![]()
Write the ionic and net ionic equations for those tests which resulted in chemical reactions.
Rxn. #3
![]()
Which of the cations could be separated from a solution containing all five using Tests #1,
#2 and #3? Could any ions be identified?
Expl. #2
![]()
Test #4
To 20 drops of each test solution add 3 drops of 6 M NaOH (dilute). If no precipitate forms skip test #5 and #6. Centrifuge, decant, and wash any precipitates that do form. Decant the supernatant liquid. Save the precipitate, free of supernatant liquid, for test #5 and #6.
Obs. #4
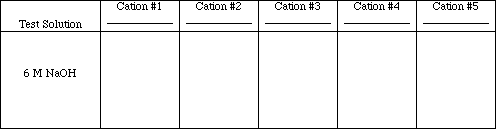
![]()
Write the ionic and net ionic equations for those tests which resulted in chemical reactions.
Rxn. #4
![]()
![]()
Test #5
![]()
Do this test in a fume hood. Add 20 drops of 15 M NH3 (concentrated) to each
precipitate from test #4 and mix. Centrifuge. Note: If no precipitate formed in Test #4 for a particular cation, place an X in the box for that ion, no test need be performed.
Obs. #5
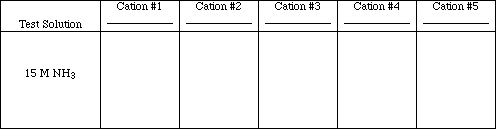
Write the ionic and net ionic equations for those tests which resulted in chemical reactions.
Rxn. #5
![]()
Test #6
![]()
Repeat test #4 to obtain new samples of the precipitates. Add 4 drops of 6 M HCl to each
precipitate and mix. Add 2 drops of 1 M NH4SCN to each test tube.
![]()
Obs. #6
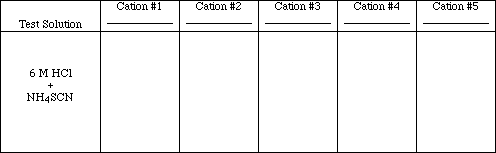
![]()
![]()
Write the ionic and net ionic equations for those tests which resulted in chemical reactions.
Rxn. #6
![]()
Which of the cations could be separated and identified from a solution containing all five
using Tests #4, #5 and #6?
Expl. #3
![]()
Using the results of your observations (Obs. #1 - #6) complete the flow chart below. Identify
the reagents at the specified points and the ions. Show your flow chart to your instructor before
continuing.
![]()
![]()
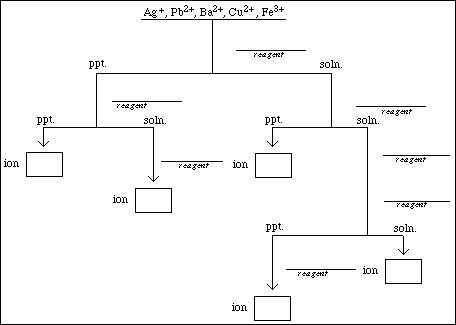
![]()
![]()
For each cation, one of the tests confirms the presence or the absence of the ion in solution. The test that produced a very dramatic change for one of the ions with little or no change for the others is the confirmatory test for that ion. Briefly summarize the confirmatory test for each ion in the space provided.
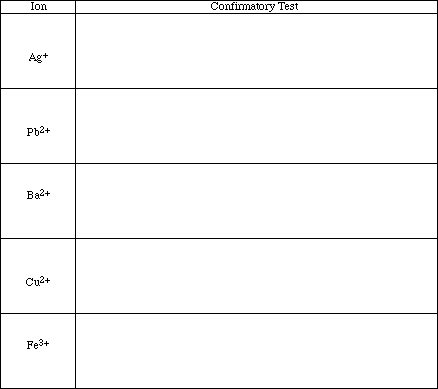
![]()
![]()
PART II: Separation of a Mixture
Prepare a mixture of all five ions by combining 1 mL samples of each 0.5 M cation solution. (To save time you may wish to approximate the 1 mL volume using a 13 x 100 test tube.) Follow your completed flow chart to confirm the presence of all five ions. Use the space below to record your observations at each step. In particular note any changes in your observations listed Obs. #1 - #6. (Note: This is a critical part of the experiment! Mixture of ions may have slightly different behavior from that of individual ions.)
![]()
![]()
PART III: Identification of Cations in an Unknown Mixture
Obtain an unknown solution. The unknown solution contains between 0-5 cations. After recording the unknown number_________ you are to analyze your unknown and identify the cations present. Additional unknown solution will be available upon request. During the analysis you are to take careful notes of the procedures you follow, recording all tests and observations. After completing your analysis, explain which cations are present and which are absent and how you arrived at these conclusions. The unknown portion of the experiment will count heavily in determining your grade so take your time and be careful.
![]()
![]()
Problems to be turned in with the laboratory write-up.
![]()
1. If you were given four unlabeled test tubes known to contain Cu(Cl)2,
AgNO3, NaCl, and
ammonia, how could you identify the contents of each test tube using no other chemical
reagents?
![]()
2. A water sample is suspected to be contaminated by iron (III) ions. How could you determine
if the sample is really contaminated? If the sample is found to be contaminated, how could you
measure the concentration of iron (III) in the sample?
![]()
Contents
![]() Return to
Return to![]() Index of Experiments
Index of Experiments
![]()