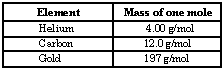Laboratory Activity: Teacher Notes
Continued
Post-Laboratory Discussion
At this point, students can begin calculations regarding molar masses of different molecules. Worksheets where "dozens" are discussed along with the concept of the mole should be provided. Throughout the year, when the mole concept or atomic masses of elements are discussed, remind students of this bean activity. Students usually make the connection easily and have a model to help them understand these ideas.
Extensions
- Ask students to weigh out 100 grains of rice, and then recalculate their relative masses. They could then rethink some of their answers about why relative masses contain the number of items they do.
- Students might also like to apply this same idea to other familiar objects- for example, game balls (baseballs, soccer balls, bowling balls, footballs, golf balls, ping pong balls, etc.) They could weigh individual balls or dozens of balls and prepare a table with names, symbols, and relative masses. Since bowling balls come in 12-pound, 16-pound sizes (and other sizes), the idea of isotopes could be discussed. The concept of a weighted average relative mass could be developed. The concept of isotopes (or relative masses) could be developed with paper clips, nails, styrofoam balls, or anything with relatively constant mass and several types of individual items.
Assessing Laboratory Learning
- The laboratory data sheets should be collected and graded. Most students will identify any large errors in measurement of relative masses because they will not fit with other answers.
- This is an opportunity to use cooperative learning and let groups of students discuss their ideas and give consensus answers to some of the more difficult questions. A group grade can then be given. Students appreciate this communication on discussion questions; cooperative learning helps foster a positive feeling about this process. It is far better than obtaining 30 papers with identical answers to discussion questions that represented original thoughts of only a few students.
- 3. Questions on worksheets as well as on tests focusing on concepts developed in this learning activity should be posed throughout this module and throughout the year. Clarity of thought on this pivotal concept should be evaluated in as many contexts as possible. (The worksheet on "dozens" uses familiar English units, so that students can work with calculations analogous to the mole concept using more familiar units. If desired, metric units could be substituted. A suitable worksheet follows.)
Answers to Worksheet on Dozens and Moles
Part I
- 1. (192 lb bowling balls) / (0.24 lb ping pong balls) = 8.0 x 10 2 times
- (1.44 lb golf balls) / (0.24 lb ping pong balls) = 6.0 times
- (0.24 lb/1 doz) x (1 doz/12 balls) = 0.020 lb 0.020 lb x (16 oz/1 lb) = 0.32 oz
- 1.44 lb x (1 doz/12 balls) = 0.120 lb 0.120 lb x (16 oz/1 lb) = 1.92 oz
- 0.040 lb x (1 doz/0.24 lb) x (192 lb/1 doz) = 32 lb
- 180 balls x (1 doz/12 balls) = 15 doz 15 doz x (1.44 lb/1 doz) = 21.6 lb
- 48 lb x (1 doz/192 lb) x (12 balls/1 doz) = 3.0 balls
- 20,000 balls x (1 doz/12 balls) x (1.44 lb/1 doz) = 2.40 x 10 3 lb
Part II
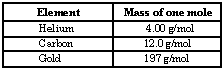
- (197 g/mol gold) / (4.00 g/mol helium) = 49.3 times
- (4.00 g/1 mol) x (1 mol/6.02 x 10 23 atoms) = 6.64 x 10 -24 g/atom
6.64 x 10 -24 g x (1 u/1.66 x 10 -24 g) = 4.00 u
- (12.0 g/1 mol) x (1 mol/6.02 x 10 23 atoms) = 1.99 x 10 -23 g/atom>
1.99 x 10-23 g x (1 u/1.66 x 10 -24 g) = 12.0 u
- 0.50 g x (1 mol/4.00 g) x (197 g/1 mol) = 25 g
(Using solution to Question 1: 0.50 g x 49.3 = 25 g)
- 3.01 x 10 24 atoms x (1 mol/6.02 x 10 23 atoms) = 5.00 mol
5.00 mol x (12.0 g/1 mol) = 60.0 g
- 25 g x (1 mol/197 g) x (6.02 x 10 23 atoms/1 mol) = 7.6 x 10 22 atoms
- 3.01 x 10 19 atoms x (1 mol/6.02 x 10 23 atoms) x (197 g/1 mol) = 9.85 x 10 -3 g
Continue