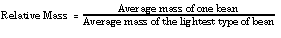Activity 1: Understanding the Mole
Activity 1: Understanding the Mole
STUDENT VERSION [Adapted from: Laboratory Manual accompanying Chemical Principles by Masterton and Slowinski. Modifications by Norma Mackenzie of Charlotte Country Day School, Charlotte, North Carolina.]
Introduction
The relative mass of an object is how many times more massive the object is than a standard object. The atomic masses of atoms are all relative masses. They can be considered relative to any particular element. Historically, both oxygen and carbon have served as the reference standard. For our purposes we can also consider atomic masses relative to the least massive element-hydrogen, with an atomic mass of approximately one. Fluorine, with a relative mass of 19, is 19 times more massive than hydrogen, etc. In this laboratory exercise you deal with the relative masses of beans. Then you will be asked to draw a parallel to the atomic masses of elements.
Purpose
To develop an understanding of the mole concept and molar masses of elements through an analogy with a model system.
Safety
Just don't spill the beans!
Part I Procedure
- Obtain a numbered plastic cup. Record the number of the cup; be sure to use the same cup during the entire activity.
- Record the mass of your cup to the nearest 0.01 g.
- Count out exactly 100 beans of one type. Discard any beans that differ greatly from an average bean. If you fail to do this, your results will not be accurate.
- Count out exactly 100 beans of one type. Discard any beans that differ greatly from an average bean. If you fail to do this, your results will not be accurate.
- Repeat Steps 3 and 4 for each type of bean provided.
Calculations
- Calculate the mass of 100 beans by subtracting the mass of the plastic cup from the mass of the plastic cup + 100 beans.
- Calculate (do not weigh) the mass of one bean of each type. Record the value in the data table. [NOTE: "Calculate" means to take the total mass of 100 beans and divide by 100 rather than weighing one particular bean.]
- Determine the relative mass of each type of bean.
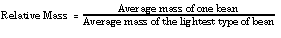
- Calculate the number of beans in one relative mass of each bean. Do this by dividing the average mass of one bean into the relative mass.

- Check your calculated results in Step 9 by following these steps:
- Weigh the empty plastic cup again and record its mass.
- Determine the sum of the relative mass of one type bean and the mass of the plastic cup.
- Place the plastic cup on the balance pan and add beans of that type until the balance contains one relative mass of that type bean. (The mass should be that calculated for the cup plus one relative mass.)
- Count the beans. Record this as the measured number of beans in one relative mass.
- Pour the beans into a pile. Retain your separate piles of relative masses of beans. You will answer questions about them later.
- Repeat for each type bean.
Continue


 Activity 1: Understanding the Mole
Activity 1: Understanding the Mole