

The mass (grams) of the substance can be converted to moles by using this relationship to construct a "unit conversion factor" and the unit-label method. Since mole is the unifying idea, if the number of molecules is desired and mass is given, mass should be converted to moles and then moles to molecules, using two factors (see transparency master).
Example: How many molecules are in 146 g HCl?

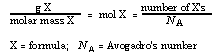

The procedure for solving empirical formula problems can be outlined using the mole concept:
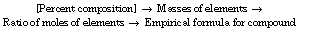
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|