14
Industrial Inorganic Chemistry (INDL)
Activity 2: The Solvay Process
Purpose
To illustrate reactions in the Solvay Process, and to produce sodium carbonate by an
industrial process.
Safety
1. Wear protective goggles throughout the laboratory activity.
2. Gloves for handling dry ice.
3. Thoroughly wash your hands before leaving the laboratory.
Procedure
Part I. Preparation of NaHCO3
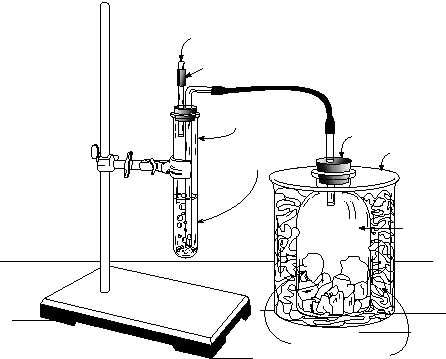
1. Obtain a 250-mL gas collection bottle and stopper, 25 X 300-mm test-tube
with a stopper assembly, and a length of rubber tubing (see Figure 1).
2. Measure 60 mL 3 M NH3 in a graduated cylinder and pour into a 250-mL
Erlenmeyer flask. Weigh out 20 g NaCl to 0.1 g and add it to the NH3 solution.
Stopper tightly, and shake the solution for several minutes to saturate it
with NaCl. If all the NaCl dissolves, add a bit more. When the solution
appears to be saturated, filter out the excess solid, using a long-stemmed
funnel and filter paper, letting the clear liquid drain into the test-tube.
3. Assemble the apparatus shown in Figure 1. The long rubber tubing should be
connected to the bent tube on the stopper assembly. The short straight tube is
fitted with a small valve, called a Bunsen valve, which will vent CO2 if the
pressure becomesexcessive.ABunsenvalveconsistsofashortlengthofrubber
tubing containing a short lengthwise slit. The valve lets gas out, but not in.
Fill the gas collection bottle
about half full with crushed
solid CO2 (dry ice). Wrap the
bottle with a towel or several
papertowelsforinsulation,and
put it into a 600-mL beaker.
Insert the stopper firmly in the
gas collection bottle.
If your apparatus is tight, you
willobservebubblesofCO2inthe
NH3-NaClsolution;theyshould
be produced at a vigorous rate,
and as they bubble up through
the solution they will react with
NH3 and become somewhat
smaller. As the reaction goes
on, the concentration of HCO3
–
ions will gradually increase.
Whilethereactionisproceeding,
begin Part II of the experiment.
However, check the stoppers
occasionally to make sure they
haven’t popped out.
LABORATORY
ACTIVITY:
STUDENT
VERSION
Figure 1. Apparatus for making NaHCO3.
Towel
Dry ice chunks
600 mL Beaker
250 mL
Gas collection
bottle
3 M NH3 saturated
with NaCl
Tight-fitting stopper
Solid glass rod
Bunsen valve
25 x 300 mm
Test-tube