Using the loop that you have made, fasten the metallic object by laying the object on the loop and draw one end of the loop through the other end. Draw the loop tightly to the object.
Figure 5. Attaching the loop to the object.
An advantage to using monofilament fishing line is that it doesnÕt absorb water when submerged under the waterÕs surface. You can use the same loop to measure all the objects whether in the air or in the water. Since the same line can be used for all weighings, the monofilament line can be tared; this increases the accuracy of measurement.
Some student groups could be assigned to do the activity by volume displacement and others by the weight displacement method to compare results. The ArchimedesÕ weight displacement method should provide better accuracy in volume measurement. Using both methods can lead to a discussion on experimental design and precision of measurement. Using the weight displacement method also can lead to a discussion of specific gravity. To illustrate specific gravity, construct a graph labelling the y-axis as volume and labelling the x-axis as (mass in air-mass in water).
If Al and Fe metal cylinders are not available, then Al and Fe bolts and screws of various sizes can be purchased in a hardware store. The volumes should range from 2-8 cm 3 . The metallic objects should be divided into two groups of 3-4 of each type of metal. Each metal type should have a full range in the volumes of the metallic objects.
Pre-Laboratory Discussion
This laboratory activity provides an opportunity for students to develop a formula from data collected in an experiment. High school mathematics classes often follow a protocol. First a formula is given. Then a table is constructed using the results of calculations from the formula. Finally, a graph is prepared from the table.
In science, the process is different. First, data are collected (often measurements) and organized into a table. Next, the data are plotted and frequently a line is drawn that Òbest fitsÓ all the plotted points. Finally, the scientist tries to make sense of the data or interpret the graph. In some instances, the scientist may derive an equation from the graph. In this laboratory activity you will try to develop a mathematical formula from collected data.
The property that will be studied has a constant value that corresponds to the slope of the line on the graph. Since the slope does not change, this property is an intensive property, one whose value does not depend upon the size of the sample.
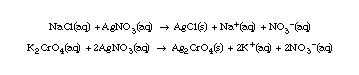
Use a beaker or flask to illustrate the above reactions. Take 10 mL 0.01 M NaCl solution; add 20 drops of K2CrO4 solution; note color. Then add the AgNO3 solution, dropwise, with swirling. Point out the color changes to the orange color that signals the end-point of the titration.
Discuss the reason for using a blank. A blank is used to correct for any chloride ion that may be present in the water being used. It normally has a value of about 0.2-0.3 mL, and must be subtracted from the amount of AgNO3 solution used in the analysis of the sample.
Make sure that students understand the proper use of burets, how to fill them, and read the volume. Stress that they must rinse the burets 4-5 times with distilled water when the laboratory is over.
Review the proper use of transfer pipets and demonstrate prior to student use.
The following "Pictures in the Mind" can be used to develop an understanding of the phenomena occurring during titration.
| TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |
|---|