Laboratory Activity: Teacher Notes
Continued
Teacher-Student Interaction
- What is the purpose of the "blank" in the titration? [The blank is necessary because some chloride may be present in the distilled or deionized water. The value normally is only 0.2-0.3 mL.]
- Why is the blank value subtracted from the total AgNO3 solution used? [The blank value is subtracted from the total AgNO3 used in order to correct for any chloride ions present in the water used to make the filtrate.]
- What are possible explanations for the chloride ion being present in the blank? [If deionized water is used, some chloride could be present from ion exchange; or glassware may not be completely rinsed.]
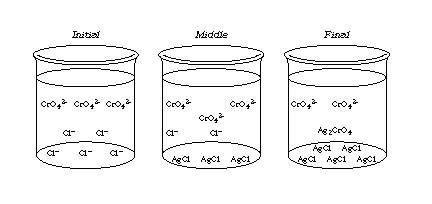
- As students titrate, ask them to notice the red Ag2CrO4 forming and then disappearing with swirling. Get them to explain the process. [The Ag2CrO4 forms and then dissolves as the AgCl precipitates preferentially because of its lower solubility.]
- Suggest that students keep the blank flask to compare with subsequent trials. This reference will help them be consistent in judging the end-point. It is helpful to keep all titration flasks lined up for color comparison. This makes it easy for the student to see if too much AgNO3 has been added.
Anticipated Student Results
- Average mL AgNO3 used for the blank = 0.3 mL
- Average mL AgNO3 used for the sample = 7.3 mL
- Mass of Fritos® used = 20.0 g
- Total volume of filtrate = 600.0 mL
Answers to Data Analysis and Concept Development
(Avg mL AgNO3 used) - (blank)/(10 mL filtrate) x (0.825 mg NaCl)/(1 mL AgNO) = (5.77 mg)/(10 mL filtrate)
(5.77 mg NaCl)/(10 mL filtrate) x (600.0 mL filtrate)/(20.0 g Fritos®) = (17.3 mg NaCl)/(1 g Fritos®)
Continue