(0.0141 mol Ag+ is approximately equal to 0.0141 mol NaCl) from equation.(0.0141 mol NaCl) x (58.5 g NaCl)/(molNaCl) = (0.825 g NaCl)/(L) = (0.825 mg/mL)
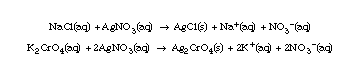
Use a beaker or flask to illustrate the above reactions. Take 10 mL 0.01 M NaCl solution; add 20 drops of K2CrO4 solution; note color. Then add the AgNO3 solution, dropwise, with swirling. Point out the color changes to the orange color that signals the end-point of the titration.
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|