Demonstration 2
Continued
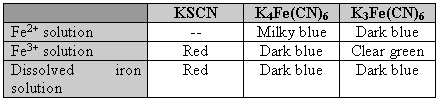
Remarks
The following questions might be used in discussion the demonstrations:
- What is the oxidation number of Fe in K4Fe(CN)6? [+3}
- Write balanced equations for each color of solution or precipitate formed in the tests.

- From your observations, draw a conclusion about the oxidation number of the iron dissolved in the HCl. Write formula(s) and name(s) for the compound(s) formed. [Both will be present: FeCl2, iron(II) chloride, and FeCl3, iron(III) chloride.]
- If iron dissolved in the stomach as slowly as it did in the beaker, would the iron in your breakfast cereal be beneficial to you? [No, it would not be in an absorbable form.]
- Of what use is iron in the body? [It is part of the heme molecule, which carries oxygen in the blood. The oxidation state of iron in the heme is +2.]
Extensions
Read other labels on vitamins, etc. to determine the form of iron present. Iron is seldom eliminated from the body. Unless you have a disease such as anemia or lose blood, you probably do not need extra iron; however, pregnant and postpartum women may need supplements.