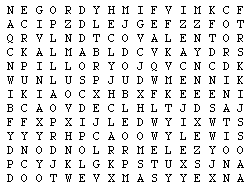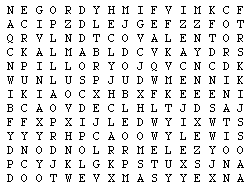
Word Search
Words about the concepts in this module can be obtained from the clues given. Find these words in the block of letters:
- Type of bond that holds metals together
- _______ bond, simultaneous attraction by two atomic nuclei for one or more electrons
- Weak intermolecular force between polar molecules
- Type of solid in which all atoms are covalently bonded
- Type of solid in which molecules are weakly attracted to each other
- Relatively strong type of bond between hydrogen and a highly electronegative atom such as O or N
- Weakest type of intermolecular attraction
- Name for electron-dot notation
- Type of covalent bond in which two pairs of electrons are shared
- Number denoting number of ions of opposite charge surrounding an ion in a crystal
Chemical Bonding (BOND)
Page 54