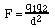
In this expression, F is the coulombic force, q is the magnitude and sign of the two charges, d is the distance separating them, and k is the proportionality constant.
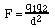
In this expression, F is the coulombic force, q is the magnitude and sign of the two charges, d is the distance separating them, and k is the proportionality constant.
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|