-
The inherent virtue of socialism
is the equal sharing of miseries;
the inherent vice of capitalism
is the unequal sharing of Budweiser.
What did Churchill actually say? (Yes, the unequal sharing of wealth!) This was, indeed, a very wise remark on Churchill's part. Some people do have more Budweiser, wealth, luck, and wisdom than do other people. Some atoms, on the other hand, are more electron-wealthy than others-they are said to be more electronegative! Fluorine is the most wealthy, the most capitalistic of all atoms. Electrons are not shared equally between atoms unless those atoms are the same, so unequal sharing is the normal situation. Capitalism reigns supreme in the atomic world!
(CHEM 13 NEWS, April 1975, p. 894)
- Bond formation can me unwell
(Electrons join up in a shell)
For it doesn't seem fine
To say they combine
When we know very well they repel!
(CHEM 13 NEWS, May 1988, p. 7)
-
While he was out walking alone,
Old Edward decided to clone.
He found himself doubled,
But not the least troubled,
For two Ed's are better than one!
-
There's a law that just simply can't be
About time and space fantasy.
But Albert Einstein
Theorized it just fine,
Because E over M is squared C.
(CHEM 13 NEWS, May 1981, p. 9)
Chemistry is full of rules
Atoms, moles, and molecules.
Some of which might fit together,
Maybe now, and likely never.
(CHEM 13 NEWS, January 1972, p. 380)
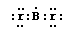 The adjacent Lewis-dot diagram for Br 2 was one of
several similar variants submitted on a recent test
by students who shall remain anonymous. (CHEM 13 NEWS, December 1991, p. 2)
The adjacent Lewis-dot diagram for Br 2 was one of
several similar variants submitted on a recent test
by students who shall remain anonymous. (CHEM 13 NEWS, December 1991, p. 2)
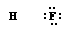
We seem to be poles apart. (CHEM 13 NEWS, September 1983, p. 15)
Beryllium gave many a plea
'Cause it's valence electrons weren't free
And it couldn't combine
till a thought came to mind
And it went and invented sp!
Boron's not satisfied yet
Its demands really cannot be met
Though it tries hard to be
Like He or Ne
sp 2 is the best it can get.
Carbon's like one seen before
His shells couldn't hold any more
Till his friends lent a hand
showed him how to expand
And sp 3 lets him join up with four!
(CHEM 13 NEWS, September 1981, p. 3)
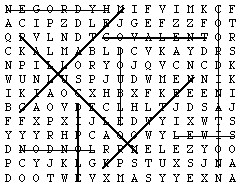
Words about the concepts in this module can be obtained from the clues given. Find these words in the block of letters:
- Type of bond that holds metals together
- _______ bond, simultaneous attraction by two atomic nuclei for one or more electrons
- Weak intermolecular force between polar molecules
- Type of solid in which all atoms are covalently bonded
- Type of solid in which molecules are weakly attracted to each other
- Relatively strong type of bond between hydrogen and a highly electronegative atom such as O or N
- Weakest type of intermolecular attraction
- Name for electron-dot notation
- Type of covalent bond in which two pairs of electrons are shared
- Number denoting number of ions of opposite charge surrounding an ion in a crystal
Answers: 1. METALLIC 2. COVALENT 3. DIPOLE 4. NETWORK 5. MOLECULAR 6. HYDROGEN 7. LONDON 8. LEWIS 9. DOUBLE 10. COORDINATION