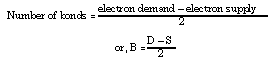
The following examples show how easy this is to use.
Determining electron dot structures for molecules is relatively easy if one knows how many bonds to draw. A powerful little bonding rule is
The following examples show how easy this is to use.
Example 1: How many bonds are present in a N2 molecule?
Step 1: Each N atom "demands" an octet (8) of electrons, so D = 2 x 8 = 16.Step 2: Each N atom "supplies" the five valence electrons it has, so S = 2 x 5 = 10.
Step 3: B = (D - S)/2 = (16 - 10)/2 = 3; there are three bonds-a triple bond. Draw the triple bond between the N atoms, then complete the octet around each N atom until all 10 "supplied electrons" are used.
Example 2: How many bonds are present in a carbonate ion, CO32- ?
Step 1: The C atom "demands" an octet (8) of electrons and each O atom "demands" an octet, so D = (1 x 8) + (3 x 8) = 32.Step 2: The C atom "supplies" the four valence electrons it has, and each O atom "supplies" six valence electrons. However, we also must consider the net ionic charge (see next step).
Step 3: Electrons have been supplied due to the 2- charge on the ion, so S = (1 x 4) + (3 x 6) + 2 = 24.
Step 4: B = (D - S)/2 = (32 - 24)/2 = 4; there are four bonds between the three oxygen and one carbon atom (from an electron-dot viewpoint, one can be regarded as a double bond; two as single bonds).
Step 5: Draw the double bond between C and one of the O atoms, then two single bonds between the C and the other two O atoms. Finally, complete the octet around each atom until all 24 "supplied electrons" are used. (NOTE: There are three different ways to draw the C = O bond, corresponding to the three resonance structures for this ion.)
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|