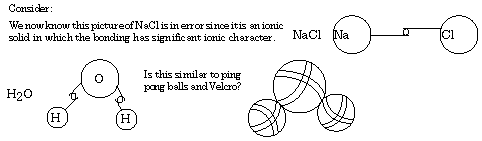
1. The development of bonding models is quite interesting when viewed from historical perspectives. When one thinks about how Dalton visualized chemically-bonded atoms, his "fishhook" model seems very simple. In view of the fact that he knew nothing about nuclei, electrons, or valences, this model is quite logical. Arrhenius presented his theory of electrolytic dissociation in 1883 to 1887, quite some time after the introduction of Dalton's ideas of bonding.
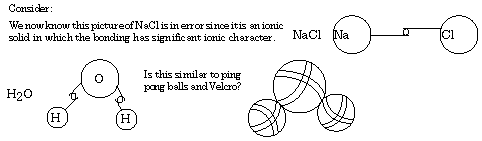
What picture does the Lewis "electron-pair" bond form? After all, we name a method of showing how the valence electrons can be arranged to explain bonding within molecules and ions. In the Lewis structure the arrangement of valence electrons can be seen around atoms in molecules and around ions in ionic compounds. Using dots to represent valence electrons, dot formulas for ions can be drawn. Remember that metals tend to lose valence electrons,3. Covalent and Polar Covalent Bonding:acquiring a 1+ charge for each electron lost. In many cases, the ions acquire a noble gas electron configuration, a configuration recognized as highly stable. This gives rise to formulas for some common metal ions of the representative elements.
In these examples, note that each formula implies an outer population of eight electrons-the original valence electrons have been lost, "uncovering" a stable octet of electrons in the next-inner shell. Such ions are generally highly stable; they each have a noble gas electron configuration. Remember that nonmetals tend to gain valence electrons, gaining a 1- charge for each electron gained. When forming anions, nonmetals tend to acquire an octet of electrons (duet in the case of hydrogen). As in the cases of metal ions, monatomic nonmetal ions thus acquire a noble gas electron configuration. Lewis-dot formulas shown below are examples of this.
Two children enter an ice cream store and pool their money to buy an ice cream cone to share. When the cone is in the possession of the first child, the second remains in the immediate vicinity and will not wander very far. This is like covalent bonding with equal sharing. If, on the other hand, one child is much larger than the other, the cone will likely be hogged by the larger one. This is like polar covalent bonding.
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|