An interaction between two atoms in which the atoms collide but do not stick together. Not a reactive collision-can be considered a "nonreactive" interaction. Physical evidence (CESK) is a weak interaction of Ha with Hb-Hc in which a collision occurs but no sticking takes place, so Ha merely rebounds. However, Hb-Hc absorbs some of the energy of the collision, so there is some physical evidence that something has happened. (Students acting out CESK should be given acting directions prior to the presentation to the class.)

*The Hb-Hc molecule looks like the same one as in Example 1, but now it has more energy and can break apart more easily into its atoms.
Close Encounters of the Third Kind: Contact
An interaction between two atoms in which the atoms not only collide but stick together to form a new molecule or ion-called a "reactive collision." Contact (CETK) is a sticky collision that forms a new chemical bond. (Students acting out CETK should be given acting directions prior to the presentation to the class.)
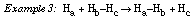
The new Ha-Hb molecule has formed! This is a successful reactive collision producing a new chemical bond.
Simply stated, if a collision is not sticky or reactive, it is not important chemically. Increasing the temperature of a chemical reaction increases the percent of effective, reactive, or sticky collisions in which new chemical bonds can be formed.
2. Orbital Overlap: A Group Activity
This activity requires two raw eggs, some vegetable oil, a hot plate, a kitchen skillet, an egg-turner, and a good-natured student willing to fry two eggs "sunny-side up." The student should be given instructions about the role to be played prior to the class period in which the activity is done. While the student is "performing", use this script:
Have you ever noticed how two eggs, sunny-side up in a skillet, may join together through their egg whites resulting in one double yoke bonded by the common white part of the two eggs? Then to break the bond between the two eggs to give one to a friend, the common white part has to be cut. Discuss several possible reasons why the eggs might run together and how this relates to what might happen between two atoms that are bonded. Is this a good analogy of a covalent bond between two hydrogen atoms? (Someone once said that there is no such thing as a perfect analogy.)
This "sunny-side up" eggs routine is a good take-off point for discussion of orbital overlap. This seems to be a good analogy for orbital overlap in terms of everyday "stuff." It should lead to a discussion of what overlap actually means-the fusion of two half-filled atomic orbitals to form a chemical bond. Most high school chemistry texts make some attempt at this explanation, thus the activity should build a mental picture that students may use to help understand orbital overlap.