 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|
A more rigorous consideration views promotion as a step in the hybridization of atomic orbitals to form equivalent bonding orbitals. Thus, in CH4, carbon's s orbital and three p orbitals are combined mathematically to form four equivalent hybrid orbitals that are used when carbon bonds with four hydrogen atoms. These four hybrid orbitals contain both s and p orbital characteristics and achieve maximum separation by pointing toward the vertexes of a regular tetrahedron at angles of 109° 29'. This is designated as sp3 hybridization since one s and three p orbitals in carbon are involved in forming four equivalent hybrid orbitals. Each hybrid orbital, like each original atomic orbital, has a two-electron capacity.All ideas needed to explain the bonding of remaining molecule-forming representative elements have now been described. In the case of boron, valence configuration 2s2 2px1 2py0 2pz0, promoting one of the 2s electrons allows formation of three equivalent bonds as in BCl3. Orbital diagrams and Lewis-dot formulas similar to those in Figures 7-10 can be drawn. In terms of hybrid bonding, three equivalent bonding orbitals designated sp2 are formed.
Beryllium reacts with hydrogen to form BeH2. Using similar reasoning to that used above, this bonding can be regarded as involving of two equivalent hybrid bonding orbitals designated as sp hybridization.
Finally, lithium reacts with hydrogen to form LiH.]
9. What is the octet rule? [When atoms react, they often change electron populations to acquire the stable electron configuration of a noble gas-eight electrons in the outer energy level. For hydrogen, of course, this would be a "duet" rule with two hydrogen atoms sharing two electrons. (This is not a hard rule since there are many exceptions. It is useful in predicting the bonding expected when many atoms form compounds. If the term "octet rule" is objectionable, an alternative is to point out that atoms tend to seek noble gas electron configurations either by electron sharing (covalent bonding) or transferring (ionic bonding) when forming compounds.]
10. In chemical reactions, do metals and nonmetals behave the same or differently with respect to sharing or transferring electrons? [Metals generally have lower electronegativities than do nonmetals. Thus, metal atoms attract electrons less strongly and tend to lose electrons to acquire an octet (noble gas electron configuration). This gives the metal atom a net positive charge, resulting in a cation. Nonmetals, on the other hand, behave in the opposite manner, having higher electronegativities than metals. Nonmetal atoms tend to gain electrons to acquire a noble gas electron configuration, giving them a net negative electric charge. Nonmetals tend to gain electrons and form negatively-charged ions (anions). These generalities hold reasonably well for many reactions involving representative elements, particularly if higher members of the carbon family are excluded.]
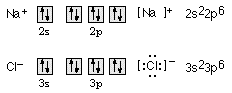
11. Draw orbital diagrams for the sodium ion, Na+, and the chloride ion, Cl-, showing the outermost energy level only. Then write the Lewis-dot formulas and electron configurations for these species... (Teacher's Note: Any simple monatomic ion will work with this question, e.g., Li+, F-, Mg2+, S2-, etc. Lewis-dot formulas of single ions are usually enclosed in brackets and the ionic charge indicated.)
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|