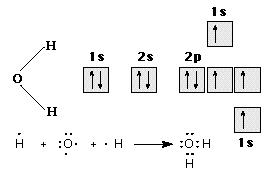
In the case of oxygen family elements (also called chalcogens), each element's atom has six valence electrons; two of these are unpaired-2s2 2px2 2py1 2pz1 in oxygen, for example. Two single covalent bonds, as for oxygen in water, may be formed under these circumstances, as illustrated in Figure 8.

Figure 8. The bonding of H2O
A similar analysis for nitrogen family elements gives three unpaired valence electrons, one in each valence shell p-orbital, providing the possibility of three covalent bonds, as in the formation of NH3 illustrated in Figure 9.
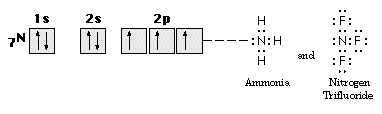
Figure 9. The bonding of NH3 and NF3.
With carbon, the situation is somewhat different. Carbon has the valence configuration 2s2 2px1 2py1 2pz0 indicating that it can form two covalent bonds. However, almost all carbon compounds form four, not two, covalent bonds. Two new ideas come into play here. First, the empty valence orbital, if CH2 formed, would give it chemical activity (reactivity) due to its close proximity to the nucleus. Second, the four equivalent bonds formed by many carbon compounds-CH4 for example-can be explained by imagining that one 2s electron is moved to the empty 2p orbital. This process, called promotion, requires energy input in a hypothetical intermediate step that is not needed in any previous cases. The energy difference between the 2s and the 2p orbitals is small, however, and does not prevent formation of four equivalent bonds. Methane, CH4, illustrates this. See Figure 10.
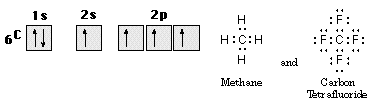
Figure 10. The bonding of CH4 and CF4.
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|