Concept/Skills Development
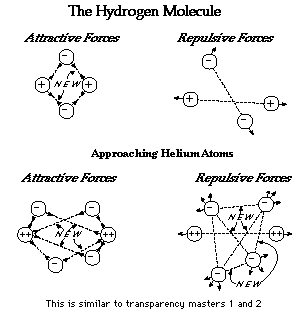 Figure 6. Bonding of the hydrogen molecule.
Figure 6. Bonding of the hydrogen molecule.
8. How many bonds may be formed by atoms of representative elements? Explain, including all groups of representative elements. [Most students will probably give the number of bonds for each periodic-group they have memorized, correlating the number of bonds with the number of valence electrons. The answer given here is more detailed than what typically good students would provided: The number of bonds that an atom of a representative element can form depends upon the orbital occupancy of valence electrons in the atom. Beginning with the halogen family, the valence electron shell has seven electrons, three pairs and one unpaired electron in s and p orbitals-3s2 3px2 3py2 3pz1 in chlorine, for example. This gives any halogen elements the possibility of forming a single covalent bond by forming one electron pair with an unpaired valence electron from another atom. This is shown by the formation of HF, illustrated in Figure 7.

Chemical Bonding (BOND)
Page 27




