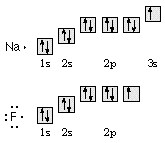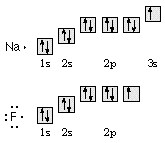
Key Questions
- What are valence electrons? [Electrons in the highest occupied energy level of an atom are known as valence electrons. These electrons determine what kind of chemical bonds, if any, the atom can form.]
- How can the total valence electrons for an element be determined? Explain. [ Electron configurations may be used to determine the number of valence electrons for an element. For example, hydrogen has only one electron-its valence electron. The configuration is 1s1. For representative elements, the number of valence electrons equals the total electron population at the highest principal energy level (n), as indicated by electron configurations.]
- How many valence electrons does a sodium, silicon, beryllium, and oxygen atom have? [sodium = 1; silicon = 4; beryllium = 2; oxygen = 6]
- What is the relation between the number of valence electrons in atoms of an element and the element's placement in the periodic table? Give examples. [The number of valence electrons determines the group placement of an element. For example, hydrogen has one valence electron; it's in the alkali metal family. All other elements in this family, Li, Na, K, Rb, and Cs, also have only one valence electron. On the other hand, fluorine has seven valence electrons, as shown by its configuration 1s2 2s2 2p5. This places it in the halogen family. (Note: This is probably more than the average student will give as an answer.)]
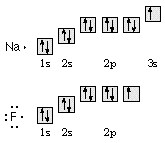
- Draw orbital diagrams for atoms of sodium and fluorine. Use the diagrams to write Lewis-dot formulas for these elements.
- Discuss how the periodic table helps to determine the number of valence electrons for an element. Include examples and state the trend in valence electrons within a group on the periodic table. [Traditional group numbers (at least as commonly used in the U.S. prior to recent international moves to re-number the groups from 1 to 18) for representative or main group elements are the key. A group IA element has one valence electron, IIA has two, IIIA has 3, and so forth. This is not surprising, since valence electrons determine chemical properties which, in turn, determine element placements in the table. For example, fluorine is in group VIIA and has seven valence electrons. All other elements in group VIIA-Cl, Br, and I-likewise have seven valence electrons. (Actual student answers may vary considerably.)]
- Why are molecules more stable than separated atoms, particularly among representative elements? [Student responses will vary from simple to complex. The following answer is more sophisticated than that expected of an average student: The origin of chemical bond stability depends upon the attractive and repulsive electrostatic forces present. Electron-nucleus interaction always furnish attractive forces while nucleus-nucleus and electron-electron interactions furnish repulsive forces. A simple way to show the origin of this stability is in terms of possible attraction for one or more electrons simultaneously by two nuclei close to each other. Whether two given atoms can form a bond depends upon the filling of orbitals in the separate atoms. Figure 6 illustrates this concept. The hydrogen molecule is used to show that when two hydrogen atoms are close together, there's a possibility of more attractive forces than repulsive forces. On the other hand, when two helium atoms approach, a net attractive force does not occur, although there are four new attractive forces. This can be explained-at least in terms of this simple analysis-by the equal number of new repulsive forces also formed, as shown in Figure 7.]
Chemical Bonding (BOND)
Page 26