 Strong bases. The strong bases are the hydroxides of the
Alkali and Alkaline Earth elements (except Be). Two common strong bases are
shown in Figure 5.
Strong bases. The strong bases are the hydroxides of the
Alkali and Alkaline Earth elements (except Be). Two common strong bases are
shown in Figure 5.
 .
..
Figure 5. Some Strong Bases.
Weak bases.Unless otherwise informed, one can
assume other common bases are weak. One example is shown in Figure 6.
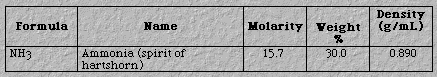 Figure 6. A Weak Base.
Figure 6. A Weak Base.
4. Equation writing and balancing
5. Stoichiometry
6. Exponents, powers of ten, logarithms
Related Skills
- Reading volumetric devices.
- Using calculator for exponents, logarithms, arithmetic operations.
- Proper handling of potentially hazardous liquids and solids. Minimum
amounts of acids and bases should be kept in the laboratory; preferably only
those amounts needed for a given activity.
Performance Objectives
After completing their study of acids and bases, students should be able to:
- define and classify acids and bases operationally and conceptually.
- write neutralization equations, given the identities of reacting acids
and bases, and complete relevant calculations based on these equations.
- identify the salt product produced in an acid-base reaction. Given a
salt,specify the acid and base from which it could be produced.
- complete an acid-base titration (small-scale or standard).
- determine whether a solution is acidic or basic using an indicator or
a pH meter.
- interpret pH values in terms of powers of ten.
- distinguish between a strong acid (or base) and a weak acid (or base)
operationally and conceptually.
- present useful mental pictures of (a) ionization, (b) dilute versus
concentrated acid/base, (c) weak versus strong acid/base, and (d)
neutralization.
- use a matrix to record and interpret experimental data.
- provide a chemical explanation for acid rain.
- express their opinions about the roles of acids and bases in the
world.
- explain how their study has changed their opinions about acids and
bases and other substances.
Acids and Bases
(Page 6)

 Strong bases. The strong bases are the hydroxides of the
Alkali and Alkaline Earth elements (except Be). Two common strong bases are
shown in Figure 5.
Strong bases. The strong bases are the hydroxides of the
Alkali and Alkaline Earth elements (except Be). Two common strong bases are
shown in Figure 5.



