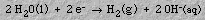
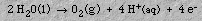
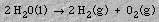
 4. Electrochemistry
4. ElectrochemistryElectrolysis of water produces an acidic solution at the anode and a basic solution at the cathode. When an electrical current is passed into graphite electrodes immersed in a sodium sulfate solution, hydrogen gas and hydroxide ions are produced at the cathode:5. Stoichiometry
At the anode, oxygen gas and hydrogen ions are formed:
The OH- and H+ ions combine to reform water. The overall reaction is
See the Stoichiometry module.6. Bonding
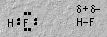
Hydrogen halides have a polar covalent bond.7. ThermochemistryIn oxyacids, hydrogen is bonded to oxygen. The central atom becomes very important in determining acidity.
Chlorine is more electronegative than sulfur and is able to weaken the O-H bond, increasing the acidity of HClO4 over that of H2SO4.
Thermochemistry is a study of the quantity of heat absorbed or evolved by chemical reactions. The heat of neutralization of a strong acid and strong base, represented by the equationis highly exothermic. Acid or base spills on the body should not be neutralized since the affected area could actually experience a heat burn due to the high heat of neutralization.
1. Environmental Science
Acid rain is rain with a pH less than 5.6. Normal rain water has a pH of 5.6 due to dissolved carbon dioxide, CO2. Acid rain is primarily a dilute mixture of sulfuric acid and nitric acid which is formed when sulfur and nitrogen oxides (nonmetal oxides) dissolve in rain or snow. Acid rain destroys lakes, kills forests and crumbles buildings and statues.Acid mine water is due to sulfuric acid produced from iron sulfide (pyrite, FeS2) in coal. Acid mine water is associated with strip mining, produced when pyrite deposits are exposed to the atmosphere. Ca(OH)2 and Al(OH)3 are important in water purification. During the settling stage, Ca(OH)2 and Al2(SO4)3 are added to produce Al(OH)3, a sticky, gelatinous precipitate that settles out slowly, carrying suspended dirt particles and bacteria with it.
 |
TABLE OF CONTENTS | TOPIC OVERVIEW | CONCEPT/SKILLS DEVELOPMENT | LINKS/CONNECTIONS | EXTENSIONS |  |
|---|