Go to Main Index
Go to Main Index Go to Main Index
Go to Main Index
Name ________________________
AP Chemistry By Satellite
John I. Gelder
Exam III
November 13, 1990
![]()
INSTRUCTIONS:
1. This examination consists of a total of 10 different pages. The last two pages include a periodic table and some useful information and a table of standard bond energies. All work should be done in this booklet.
2. PRINT your name, high school, teaching partner's name and the date now in the space at the top of this sheet. DO NOT SEPARATE THESE PAGES.
3. Answer all questions that you can and whenever called for show your work clearly. Your method of solving problems should pattern the approach used in lecture. You do not have to show your work for the multiple choice or short answer questions.
4. No credit will be awarded if your work is not shown in problems 2a, 2d, 2e, and 11.
5. Point values are shown next to the problem number.
6. Budget your time for each of the questions. Some problems may have a low point value yet be very challenging. If you do not recognize the solution to a question quickly, skip it, and return to the question after completing the easier problems.
7. Look through the exam before beginning; plan your work; then begin.
![]()
8. Relax and do well.
![]()
APCBS Exam III KEY PAGE 2
![]()
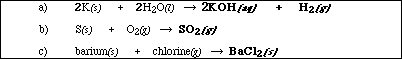
(9) 1. Write the chemical formula(s) of the product(s) and balance the following reactions. Identify all
products phases as either (g)as, (l)iquid, (s)olid or (aq)ueous.
(16)2a. Using Bohr's model, calculate the energy of the first three levels in the hydrogen atom.

b. Sketch an energy level diagram and label the three energy levels with the corresponding value of the principle quantum number.
![]()
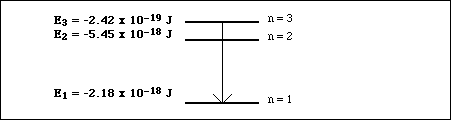
c. In the energy level diagram above, draw an arrow from the starting energy level to the ending energy level to indicate the electron transition which would result in the emission of a photon of light having the greatest possible energy.
d. Calculate the energy of the photon of light emitted in part c.

APCBS Exam III KEY PAGE 3
![]()
e. Calculate the wavelength of the photon in part d.
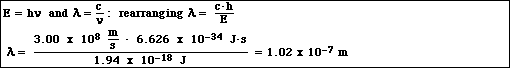
![]()
(6) 3. Sketch the basic shape of an s orbital, a p orbital and any d orbital.
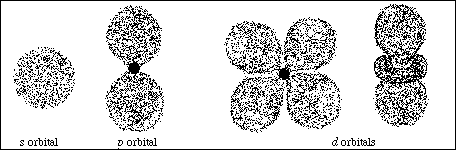
(6) 4. Predict the formula of a compound which contains the following elements and name each compound.

(4) 5. Short answer.
a) How many orbitals in the l = 1 subshell?
The l = 1 subshell is also called the p subshell. All p subshells contain 3 orbitals.
b) What is the maximum number of electrons in a d subshell?
All d subshells contain 10 electrons.
c) What is the subshell designation when n = 3 and l = 2?
The l = 2 subshell is also called the d subshell. So the subshell designation is 3d.
d) How many subshells in the n = 4 shell?
The n = 4 shell contains 4 subshells.
APCBS Exam III KEY PAGE 4
![]()
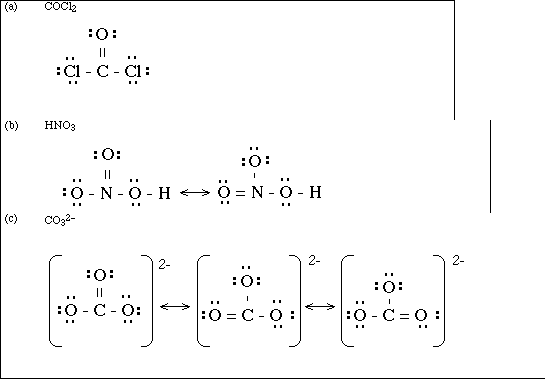
(15) 6. Draw a Lewis electron-dot structure for each of the covalent molecules below. Include all resonance
structures if they are needed to adequately represent the bonding in the molecule.
(6) 7. Write the electron configuration for each of the following.
![]()
a) Cu [Ar] 4s 13d10 or 1s 22s 22p63s 23p64s 13d10
![]()
b) Ca2+ [Ne]3s 23p6 or 1s 22s 22p63s 23p6
![]()
c) Po [Xe] 6s 24f145d106p4 or 1s 22s 22p63s 23p64s 13d104p65s 24d105p66s 24f145d106p4
![]()
(2) 8. Sketch the orbital diagram for the valence electrons in copper.
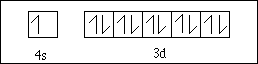
APCBS Exam III KEY PAGE 5
![]()
(10) 9. The plot of the first ionization energy of the elements in the 1st period is shown below.
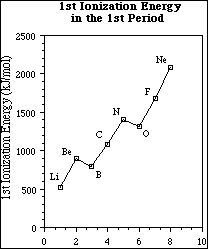
a) Identify the overall trend in the first ionization energy proceeding from lithium to neon.
The first ionization energy increases from lithium to neon.
b) Briefly, explain the reason for this overall trend.
Moving across the period protons are added to the nucleus and electrons are added to the n = 2 level. Electrons in the same level do not shield each other as effectively as do inner shell electrons. The increase in protons causes the positive charge seen by the valence electrons, the effective nuclear charge, to increase with increasing atomic number. The increased effective nuclear charge means the valence electrons are attracted more strongly from lithium to neon.
c) Explain the observed exception to the overall change in ionization energy when going from nitrogen to oxygen.
Oxygen has a lower first ionization energy because the electron being removed is a paired 2p electron. This electron experiences greater electron-electron repulsions than the unpaired electrons in the same sublevel, and therefore, less energy is required to remove the electron.
APCBS Exam III KEY PAGE 6
![]()
(8) Solve ONE of the two problems on this page. (A second problem will not be scored.)
10. In Experiment #5 measurements were made on the three reactions,
![]()
and the results were used to verfiy Hess' law. Briefly tell what measurements were made and how Hess' law was verified.
![]()
In Experiment #5 the temperature change of the solutions in which the above reactions
occurred was measured. Knowing the mass of the solution, the specific heat of the
solution and the heat capacity of the calorimeter, DHrxn for each reaction was
calculated.
![]()
Hess' law was verified by adding the second and third equation and calculating the
DHrxn for the resulting equation. This value was then compared to the measured
DHrxn for the reaction. Hess' law was verified when the two values were found to be
very close in magnitude.
![]()
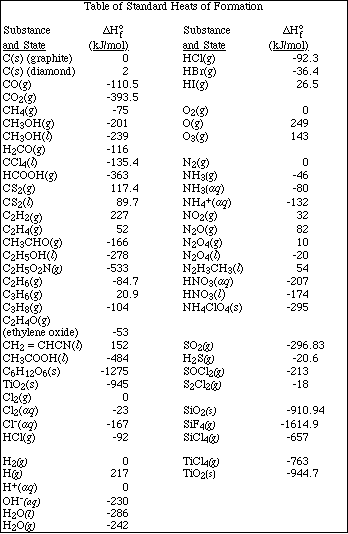
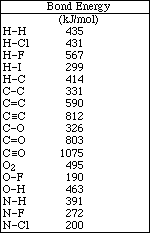
11. Calculate the DHrxn for the following reaction using the table of bond energies.
![]()
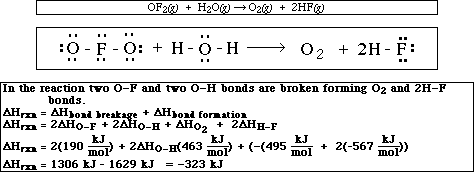
APCBS Exam III KEY PAGE 7
Multiple Choice:
Print the letter (A, B, C, D, E) which corresponds to the answer selected.
12. C ![]() 13. D
13. D ![]() 14. C
14. C ![]() 15. B
15. B
16. B ![]() 17. E
17. E ![]() 18. A
18. A ![]() 19. D
19. D ![]() 20. B
20. B
ONLY THE ANSWERS IN THE AREA ABOVE WILL BE GRADED. Select the most correct answer for each question. Each question is worth 2 points.
12. Which of the examples of electromagnetic radiation is lowest in energy?
A) X-rays
B) red light
C) infrared radiation
D) green light
E) ultraviolet radiation
13. Which of the following has the largest radius?
A) Cl-
B) Na+
C) Al
D) S2-
E) H+
14. The first five ionization energies, in kJ/mol, for a particular element are,
![]()
![]()
What is the most stable charge on the element?
A) +1
B) +2
C) +3
D) +4
E) +5
15. Which quantum number determines the orbital shape?
A) n
B) l
C) ml
D) ms
E) none of the quantum numbers determine orbital shape.
APCBS Exam III KEY PAGE 8
![]()
16. Which of the following sets of quantum numbers is not allowed for an electron in a hydrogen atom?
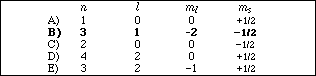
17. Which of the following elements has the largest electronegativity?
A) Ba
B) Ca
C) He
D) Br
E) O
18. An atom of the element X has the electron configuration
1s22s22p63s23p4
The compound most likely to form with Ca is,
A) CaX
B) CaX2
C) Ca2X
D) Ca2X3
E) Ca3X2
![]()
19. Which of the following orbital diagrams violates Hund's rule?
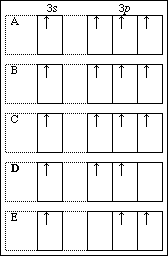
APCBS Exam III KEY PAGE 9
![]()
20. Which of the following represents the ground state electron configuration for Ni2+? (Atomic number of
Ni is 28)
A) [Ar]4s23d8
B) [Ar]3d8
C) [Ar]4s23d6
D) [Ar]3d10
E) [Ar]4s13d7