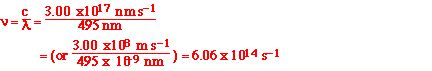2. Answer the following questions regarding light and its
interactions with molecules, atoms and ions.
(a) The longest wavelength of light with enough energy to
break the Cl-Cl bond in Cl2(g) is 494 nm.
(i) Calculate the frequency, in s-1, of
the light.
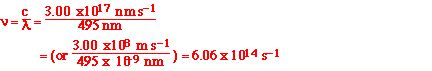
(ii) Calculate the energy, in J, of a photon of the
light.
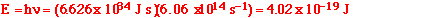
(iii) Calculate the minimum energy, in kJ mol-1,
of the Cl-Cl bond.
(b) A certain line in the spectrum of atomic hydrogen is
associated with the electronic transition in the H atom from
the sixth energy level (n = 6) to the second energy
level (n = 2).
(i) Indicate whether the H atom emits energy or
whether it absorbs energy during the transition. Justify
your answer.
The n = 6 state is
higher in energy than the n = 2 state. Going
from a high energy state to a low energy state means that
energy must be emitted.
(ii) Calculate the wavelength, in nm, of the
radiation associated with the spectral line.

(iii) Account for the observation that the amount of
energy associated with the same electronic transition (n
= 6 to n = 2) in the He+ ion is
greater than that associated with the corresponding
transition in the H atom.
The positive charge holding the
electron is greater for He+, which has a 2+
nucleus, than for H with its 1+ nucleus. The stronger
attraction means that it requires more energy for the
electron to move to higher energy levels. Therefore,
transitions from high energy states to lower states will
be more energetic for He+ than for H.