Problem I:
Calculate the molality and mol fraction
of HCl for a solution which has a mass percent of 37.1 %.
Answer:
This problem is a little different compared
to problems we've done in class. I did not tell you how much of the solute and
the solvent is available, I just gave you the percentage by weight of the solute
HCl.
But that is OK we can assume any amount
of solute and solvent and still get the correct answer.
So you assume an amount of solute and solvent,
but just remember is must be 37.1% by weight HCl.
Did you come up with 37.1 grams of HCl
and 62.9 grams of H2O?
This is how the expert thinks....If the
solution is 37.1% by weight HCl according to the definition of weight % that
means in 100 grams of solution there must be 37.1 grams of HCl. If there is
100 grams of solution and only HCl and H2O are present than 100
grams - 37.1 gram = 62.9 grams H2O.
We were not given an amount of HCl or H2O
so we can make up how much we want! The easiest way is to assume an amount in
terms of the weight % definition. Click here for a quick
text of this logic.
Now we can calculate the molality of HCl
in the solution.
What is the definition of molality?
Molality is defined as the mols of solute
per kilogram of solvent.

Given the mass of solute and solvent we
have assumed for this problem, calculate the mols of solute (HCl) and the kilograms
of solvent (H2O).
We just need to use the molar mass of HCl
and the conversion from grams to kilograms for H2O. As shown below;
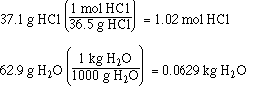
Now calculate the molality of the solution.
The molality of the solution is the quotient
of the mols solute and the kilograms of solvent.
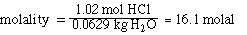
Now calculate the mol fraction of HCl in
the solution.
The definition of mol fraction in this problem is;
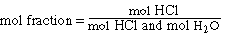
We know the mols of HCl in the solution from the calculation
of the molality, the mols of H2O is calculated
as;
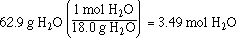
With this information calculate the mol fraction of HCl in the
solution.