How much heat is required to change 36.0 g of H2O(l) at 100 ūC
to 36.0 g of H2O(g) at 100 ūC?
Answer;
I got 91.5 kJ. Is that what you got? Here is how I solved the problem.
Solution:
In this type of problem the best approach is to determine each of the steps
along the heating curve required to effect the change;
Step 1) : H2O(s) at -10 ūC to H2O(s) at 0 ūC
Step 2) : H2O(s) at 0 ūC to H2O(l) at 0 ūC
Step3) : H2O(l) at 0 ūC to H2O(l) at 100 ūC
Step 4) : H2O(l) at 100 ūC to H2O(g) at 100 ūC
Step 5) : H2O(g) at 100 ūC to H2O(g) at 110 ūC
Calculations;
Step 1) :
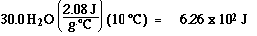
Step 2) :
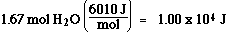
Step3) :
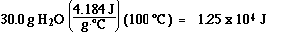
Step 4) :
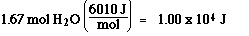
Step 5) :
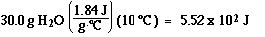
TOTAL 9.15 x 104 J (91.5 kJ)