A titration is performed using by adding 0.100 M NaOH to 40.0 mL of 0.1 M
HCl.
e) Calculate the pH after addition of 10.0 mL of 0.100 M NaOH past the endpoint.
Answer:
When a strong base like NaOH is added to a strong acid like HCl a neutralization
reaction occurs,
NaOH(aq) + HCl(aq) ---> NaCl(aq) + H2O(aq)
The K for this reaction is very large,
so the reaction goes to completion. To determine how much reaction occurs we
need to set up an ICE 'like' table.
|
NaOH(aq)
|
+ HCl(aq)
|
--->
|
NaCl(aq)
|
+ H2O(aq)
|
I
|
|
|
|
|
|
C
|
|
|
|
|
|
F
|
|
|
|
|
|
Determine the moles of NaOH (that were added) and the moles of HCl (present
initially);
The moles of NaOH added from the buret are;
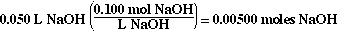
The volume of 50.0 mLs was obtained by adding 10.0 mLs (volume
past the equivalence point) plus the 40.0 mLs of NaOH equired to reach the equivalence
point.
The moles of HCl in the flask initially are;
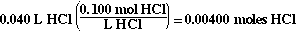
So the 'ICE table' now looks like;
|
NaOH(aq)
|
+ HCl(aq)
|
--->
|
NaCl(aq)
|
+ H2O(aq)
|
I
|
0.00500 mol
|
0.00400 mol
|
|
0
|
-
|
C
|
|
|
|
|
|
F
|
|
|
|
|
|
Since K for the reaction is large, the reaction will go to
completion. This means that the reactant in the smallest amount will completely
react. At this point in the titration there are fewer moles of base compared
to acid. So all the base reacts. Adding this to the 'ICE table' we have;
|
NaOH(aq)
|
+ HCl(aq)
|
--->
|
NaCl(aq)
|
+ H2O(aq)
|
I
|
0.00500 mol
|
0.00400 mol
|
|
0
|
-
|
C
|
-0.00400 mol
|
-0.00400 mol
|
|
+0.00400 mol
|
-
|
F
|
|
|
|
|
|
The final amount of each species can be obtained just as it
always has been in the other ICE tables, by adding the change to the initial
amount.
|
NaOH(aq)
|
+ HCl(aq)
|
--->
|
NaCl(aq)
|
+ H2O(aq)
|
I
|
0.00500 mol
|
0.00400 mol
|
|
0
|
-
|
C
|
-0.00400 mol
|
-0.00400 mol
|
|
+0.00400 mol
|
-
|
F
|
0.00100 mol
|
0
|
|
+0.00400 mol
|
|
Focusing on the Final row there is base remaining and all the
acid has reacted. Salt has been formed.
To calculate the pH we must recognize the type of system we
have. To do that we look at the final row and see what species are present.
In this case there are moles of a strong base (NaOH) and its salt (NaCl).
But the salt of a strong acid and a strong base does not effect the pH of
water, so we left with only moles of NaOH in the mixture.
To calculate the pH of the solution we must determine the concentration
of NaOH in the mixture. The concentration is obtained by dividing the volume
of the solution into the moles of NaOH. The volume of the solution is 90.0
mLs (40.0 mLs of HCl and 50.0 mLs of NaOH).
So the [NaOH] is;
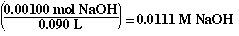
Solving for the pOH of a strong base;
pH = - log [OH-]
Since strong bases completely dissociate the [OH-]
= [NaOH] = 0.0111 M
pOH = - log 0.0111
pOH = 1.95
pH = 14.0 - 1.95 = 12.0
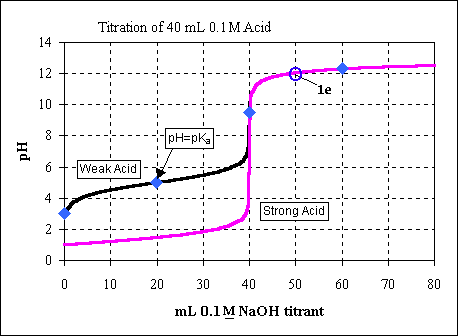
We can see this point on the titration curve (as point 1e).