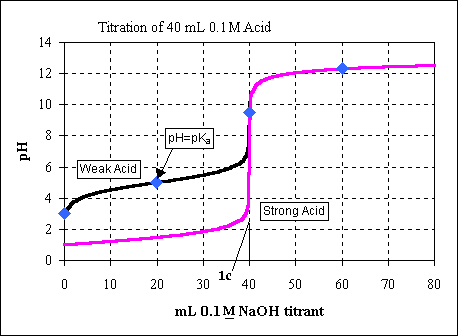
A titration is performed using by adding 0.100 M NaOH to 40.0 mL of 0.1 M
HCl.
c) Calculate the volume of base needed to reach the equivalence point.
Answer:
The equivalence point is the point in the titration where;
moles of base (NaOH) = moles of acid (HCl)
Since we are adding the base to the acid, we know how many moles
of acid are present;
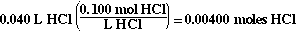
We need the same number of moles of base (NaOH) at the equivalence
point;
0.00400 moles NaOH
Knowing the concentration of the NaOH we can use the moles of
NaOH required to reach the equivalence point to determine the volume;

So 0.0400 L or 40.0 mLs of 0.100 M NaOH are required to reach
the equivalence point in this titration.
We can see this point on the titration curve (as point 1c).