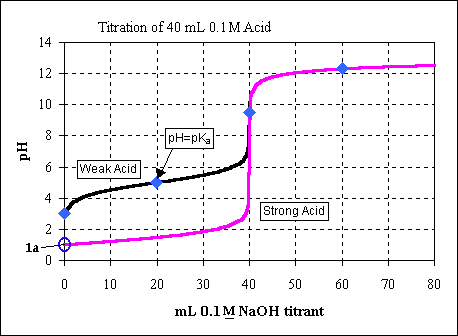
A titration is performed using by adding 0.100 M NaOH to 40.0 mL of 0.1 M
HCl.
a) Calculate the pH before addition of any NaOH.
Answer:
This first calculation is asking for the pH of the solution of the strong
acid before adding any base. The concentration of the HCl before adding base
is 0.100 M. For this first calculation we do not care what volume of the acid
we have. All we need to do is solve for the pH of a strong acid. Solving for
the pH of a strong acid only requires using the equation;
pH = - log [H+]
Since strong acids completely dissociate the [H+]
= [HCl] = 0.100 M
pH = - log 0.100
pH = 1
We can see this point on the titration curve (as point 1a).