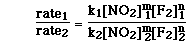
Let's consider the following problem;
The following initial rate data were collected for the reaction
at 100 degrees C.
| Experiment # | [NO2] | [F2] | Initial Rate (M/min) |
| 1 | 0.0482 M | 0.0318 M | 1.9 x 10-3 |
| 2 | 0.0120 M | 0.0315 M | 4.69 x 10-4 |
| 3 | 0.0480 M | 0.127 M | 7.57 x 10-3 |
i) Determine the reaction order for NO2(g) and F2(g).
To determine the order with respect to NO2(g) the ratio of experiments 1 and 2 must be used. Even though the concentration of F2(g) is not exactly constant, it is to two significant figures.The ratio of the two experiments can be written as;
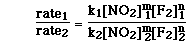
Since the rate constant is constant and the concentration of F2(g) is constant we can cancel both of those terms and substitute for the initial rate and concentration of NO2(g). We then have;

Reducing the two ratio yields;
So the order of the reaction with respect to NO2(g) is 1.
To determine the order with respect to F2(g) the ratio of experiments 1 and 3 can be used. Even though the concentration of NO2(g) is not exactly constant, it is to two significant figures.
The ratio of the two experiments can be written as;
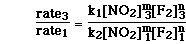
Since the rate constant is constant and the concentration of NO2(g) is constant we can cancel both of those terms and substitute for the initial rate and concentration of F2(g). We then have;

Reducing the two ratio yields;
So the order of the reaction with respect to F2(g) is 1.