 Concentration
Concentration Surface Area
Surface Area
Chemical Kinetics concerns itself with speed at which chemical reactions occur and what factors effect the speed of the reaction. To study the speed of a chemical reaction we must follow the rate which reactants disappear or products appear.
In class we explored the factors which effect the speed of a chemical reaction;
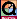 Concentration
Concentration Surface Area
Surface Area magnesium and different concentrations of hydrochloric acid. The equation which describes the reaction is;
magnesium and different concentrations of hydrochloric acid. The equation which describes the reaction is;
We did not do a demonstration of how temperature effects the rate of a chemical reaction, but we discussed how increasing the temperature of a chemical reaction increases its rate.
To study how a catalyst effects the rate of a reaction we did the "Elephant Toothpaste" demonstation. In this demonstration we observed how the rate of decomposition of hydrogen peroxide is effected by the presence of the catalyst potassium iodide. The decomposition reaction is;
To demonstrate the effect of surface area on the rate of a chemical reaction we tried to burn a small pile of lycopodium powder and then we observed the effect of spraying the powder into the flame of a Bunsen burner.
A chemist's goal of studying how the above factors effect the rate of a chemical reaction is to understand the details of how the reactants are converted to products from a molecular view. That is, to use experimental evidence and chemical intuition to produce a reaction mechanism which describes the stepwise process which is believed to convert reactants to products.
Chemical kinetic concerns itself with what happens while a reaction is underway, not just in the final outcome. Chemical kinetics does not change the chemical reaction it simply provides a clearer picture of what happens during the progress of the chemical reaction. With the new knowledge it will be possible to control the conditions of a reaction which will help increase the amount of the desired products. If we were able to travel to the molecular level and watch the progress of the reactants in the course of the reaction and the many changes they undergo in proceeding to the products we would be doing chemical kinetics.
But, unfortunately, we can not 'see' molecules or electrons with our own eyes so it is impossible to view these molecular interactions firsthand. What we do instead is to use instruments that follow the reaction, by watching properties of particular reactants that depend upon their concentration. This way we can watch how a reactant disappears in a chemical reaction. Or we can watch the properties of a particular product that depend upon concentration and we can see how its concentration changes during the course of a chemical reaction. Then by applying mathametical models and doing some calculations it is possible to understand the pathway a reaction follows.
To better understand the factor which effect the rate of a chemical reaction our first step is to understand the term reaction rate. To begin we must discuss the term rate. Rate is a measure of the speed of a process and is usually describe as a ratio of an event with time. The speed that we drive an automobile is expressed in miles per hour. Filling a sink or bathtub with water from a faucet. The faucet delivers water at a rate of gallons per minute. Rate in each case is similar in that it is a change in a quantity with time.
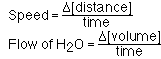
For a chemical reaction its rate, rate of reaction, is expressed in terms of how fast the concentration of a substance changes in the course of a chemical reaction.

For the reaction
the rate can be expressed in terms of the reactant or any of the products.