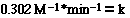So from the two experiments lets summarize the important information.
We did the same reaction for each experiment, at the same temeprature and other
conditions. The only thing different between the two experiments was the initial
concentration of [NO2].
Experiment
|
[NO2]
|
Initial Rate (M min-1)
|
1
|
0.350 M
|
3.7 x 10-2 M min-1
|
2
|
0.700 M
|
1.5 x 10-1 M min-1
|
The data clearly says the initial rate of the reaction is dependent
on the initial concentration of NO2. From this knowledge of the dependence
of the initial rate of a reaction on the initial concentration of NO2
it is possible to determine the rate equation for the reaction. The rate equation
gives the mathematical relationship between the reaction rate and the concentration
of one or more reactants. Here is an animation
of the data and how to obtain an average, instantaneous and initial rate.
From the data we see going from Exp 1 to Exp 2 we doubled the
[NO2].
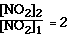
Whan happened to the ratio of the initial rates?
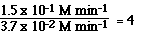
Had we tripled the initial concentration of NO2 the
initial rate would have increased by a factor of nine.
So how do we interpret this information...;
[NO2] increase by factor of 2 the rate increases
by a factor of 4
[NO2] increase by factor of 3 the rate increases
by a factor of 9
So the rate increases by the square of the concentration. We
could write a relationship mathematically that reflects the experimental data;
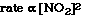
The exponent used with the [NO2] is called the order
for NO2. We say the order of the reaction with respect to NO2
is 2. Or we say the reaction is second order in NO2.
To convert the relationship from a proportionality to an equality
we need to add a constant. We will use 'k' to represent the constant and we
will call the constant the rate constant. The new equation now becomes;
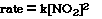
This is called the rate law equation
for the reaction. It reflects the relationship betwen the initial rate, the
rate constant and the order for each reactant.
For this reaction, under these experimental condition we need
to know what the magnitude and the units of k, the rate constant. To determine
the rate constant for the reaction we substitute
for the [NO2] and the initial rate from either of the experiments.
So;
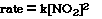
Substituting for Experiment 1

and