Ionic Radii
Before continuing we should consider the radii
of the ions (common cations/common anions) formed when
electrons are lost or gained by neutral atoms. The cation formed
when one or more electrons are lost has a smaller ionic radius
compared to the neutral atom. The loss of electrons increases the
effective nuclear charge of the electrons remaining on the ion
and they experience a greater attraction towards the nucleus.
Adding of one or more electrons to a neutral atom, to form an
anion, decreases the effective nuclear charge of the valence
electrons and the ionic radius is greater compared to the neutral
atomic radius. For isoelectronic ions the greater the positive
charge the smaller the ionic radius. While for the isoelectronic
ions the greater the negative charge the larger the atomic
radius.
Nuclear Charge
|
Element
|
Electronic Configuration
|
Effective Nuclear Charge
|
Valence Electrons
|
Core Electrons
|
4+
|
Be
|
1s22s2
|
2+
|
2
|
2
|
5+
|
B
|
1s22s22p1
|
3+
|
3
|
2
|
5+
|
B+
|
1s22s2
|
3+
|
2
|
2
|
10+
|
Ne
|
1s22s22p6
|
8+
|
8
|
2
|
9+
|
F
|
1s22s22p5
|
7+
|
7
|
2
|
9+
|
F–
|
1s22s22p6
|
7+
|
8
|
2
|
Same number of valence electrons in Be and B+,
however the valence electrons in B+ 'see' a greater
effective nuclear charge and are attracted closer to the
nucleus––smaller radius than Be.
Same number of valence electrons in Ne and F–,
however the valence electrons in F– 'see' a
smaller effective nuclear charge and are not held as tightly by
the nucleus––larger radius than Ne.
Some elements which form ionic compounds are too far removed
from a noble gas to readily achieve the rare gas configuration.
Members of the transition metals series would have to lose a
large number of electrons to become isoelectronic with the
nearest noble gas. Most transition metals form cations of 2+ and
3+. This suggests that stable ions can exist which do not
duplicate the electronic arrangements of the noble gases. Some of
the ions can be characterized by filled subshells, for example,
Zn2+ [Ar]3d10, Ag+ [Kr]3d10
and Pb2+ [Xe]6s25d10. It is
therefore more difficult to predict most stable ions in the
transition metals. Instead their chemistry is a collection of a
large variety of ionic compounds.
Covalent Compounds
As important and varied as ionic compounds are, there are
many compounds which do not demonstrate the high melting points,
high water solubility and electrical conductivity of ionic
compounds. This other group of compounds consists of examples
that are gaseous, liquids and solids at room temperature, and
generally they have low melting or boiling points. Frequently
they are insoluble in water. They do not conduct electricity in
the liquid state, or when soluble in water, do not conduct
electricity in aqueous solution. Compounds that do not conduct
electricity are called nonelectrolytes. There is no
evidence to indicate that the elements in these compounds are
ionic. Rather there is considerable experimental evidence to
suggest that such compounds are made of discrete molecules, hence
compounds in this group are called molecular compounds.
The forces that hold the atoms together in molecular
compounds can not be understood on the basis of oppositely
charged ions. So it was that Gilbert Lewis proposed that the
strong attractive force between two atoms in a molecule reselted
from a covalent bond, formed by sharing of a pair
of electrons between the atoms in the bond.
In hydrogen, H2, as the two hydrogen atoms
approach one another their spherical
1s orbitals begin to overlap. Each electron occupying the
space around the two nuclei. Each electron is attracted
simultaneously by each nuclei. The attraction that bonds the
electrons to both nuclei is the force holding the atoms together.
Thus while ions do not exist in covalent compounds the bond can
be regarded as arising from the attraction of oppositely charged
particles–nuclei and electrons.
We can represent the formation of the covalent bond in
hydrogen by writing the Lewis electron–dot formula
for the atoms and the molecule.
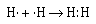
The two electrons between the two hydrogen nuclei represent a
covalent bond. And the two electrons are referred to as an
electron pair. Each hydrogen atom can be thought of as
sharing the pair of electrons. When we think in these terms we
note that each atom has a 1s2 electron configuration,
isoelectronic with the next noble gas–helium.
The formation of the covalent bond in gaseous HF can be
described in similar terms;
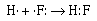
The fluorine atom and the hydrogen atom each require one
electron to achieve a configuration isoelectronic with a noble
gas. Helium for hydrogen and neon for fluorine. The two atoms
share the electron pair to achieve their respective noble gas
configurations. The other three pairs of electrons around the
fluorine atom are called nonbonding or lone pair
electrons.
We can understand the formulas of a large number of covalent
compounds by writing or drawing the Lewis electron–dot
formulas for the compounds.
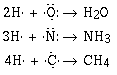
(Use lines to denote the covalent bond and dots to denote
the electron pairs.)
Notice that in writing our formulas that F, N, O and C all
have eight electrons around it. The tendency of atoms in a
molecule to share electrons to have a total of eight is called
the octet rule. Many compounds follow the octet rule, and
some do not.
So far all the examples we've discussed involved atoms
sharing two electrons. Two electron bonds are called single
bonds. However, it is possible for two atoms to share two
pairs of electrons to form double bonds and even three
pairs of electrons to form triple bonds. Atoms such as C,
N, O and S form double bonds in certain instances, while C and N
exhibt examples of triple bonds.
Although the covalent bonds in dihydrogen, H2,
and hydrogen fluoride, HF, each involve a pair of electrons
being shared, there are some important differences in the way
inwhich the electrons are shared. In hydrogen it can be noted
that the electrons spend an equal amount of time near each
nucleus. However, it can not be said that the electron pair
shared between hydrogen and fluorine in HF is equally shared. In
fact, the electrons spend more time near the fluorine nucleus
than near the hydrogen nucleus. Resulting in the fluorine
appearing to have some extra negative charge and the hydrogen
some extra positive charge.
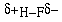
The covalent bond in HF is described as a polar covalent
bond because the electrons spend an unequal amount of time on
the two nuclei. The bond in hydrogen is called a nonpolar bond.
It should be noted that the polar bond can be viewed as an
intermediate case between the equal sharing of electrons in
hydrogen and the transfer of electrons that arises in ionic
bonds.
Atoms form compounds by losing, gaining or sharing enough
electrons to achieve the outer electron configuration of a noble
gas. The combining capacities of atoms are a consequence of the
proportions in which they must associate to achieve noble gas
configurations.
ELECTRONEGATIVITY
Electronegativity is a concept which is used to help
understand which atom in a chemical bond will have the greatest
attraction for the bonding pair of electrons. Electronegativity
is a measure of the ability of an atom in a chemical bond to
attract electrons to itself.
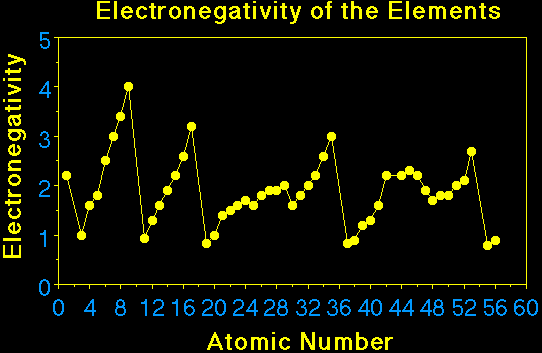
From the figure one can see the important trend in
electronegativity across a period and down a group. In general
the electronegativity increases going across a period, and
decreases going down a group. The larger the electronegativity
value the greater the attraction of the electron by the atom. The
absolute value of the difference in electronegativity of two
atoms sharing a pair of electrons is a measure of the degree of
polarity of the chemical bond. When the difference is small the
bond is nonpolar. When it is large the bond is polar, if the
difference is very large the bond is ionic.