Calculate the final temperature when a 136 mL of H2O at 25.0 C
is mixed with 75.0 mL of H2O at 82.0 C.
Solution:
The important thing to remember here is when the two samples of water are
mixed the sample at the higher temperature will transfer heat to the sample
at the lower temperature. We can write this mathematically as,
qhot water = -qcold water
The amount of heat transferred is determined by the equation;
q= S.H. * mass * 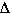 T
T
So substituting this relationship into the first equation we get,
S.H.hot water * masshot water * 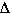 Thot
water = - S.H.cold water * masscold water *
Thot
water = - S.H.cold water * masscold water * 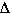 Tcold
water
Tcold
water
Now we simply substitute the information we know, (remember the
density of water is 1.00 g mL-1
1.84 J·g-1C-1
* 136 g * 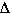 Thot water
= - 1.84 J·g-1C-1 * 75.0
g *
Thot water
= - 1.84 J·g-1C-1 * 75.0
g * 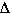 Tcold water
Tcold water
This can be reduced to,
1.81 (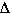 Thot
water) = -
Thot
water) = - 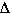 Tcold water
Tcold water
Remember that,
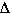 T
= Tfinal - Tinitial
T
= Tfinal - Tinitial
Therefore,
1.81 (Tfinal - Tinitial)hot = - (Tfinal - Tinitial)cold
Substituting;
1.81 (Tfinal - 25 C)hot =
- (Tfinal - 82.0 C)cold
1.81Tfinal - 45.25 = - Tfinal + 82.0
C
2.81Tfinal = 127.2
Tfinal = 45.3 C