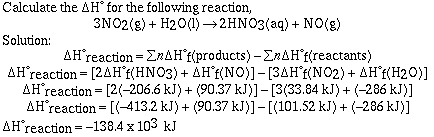We have discussed how we use calorimetry as an experimental method to determine
the heat released or absorbed by a chemical reaction. I demonstrated both the
coffee-cup and the bomb calorimeter. The coffee-cup calorimeter measures the
heat released or absorbed in a reaction occuring in aqueous solution. I did
one example showing how to calculate the heat of a reaction knowing the temperature
change, the mass of the water and the heat capacity of the calorimeter. The
bomb calorimeter measured the heat released in a combustion reaction. Bomb calorimeters
are used to measure the heat of a reactions involving gases.
I made two important points during the lecture which I did
not dwell on then, but which I want to re-iterate now. There is
an important difference in the heat transferred in the two
different types of calorimeters. The coffee-cup calorimeter
measures the heat of a reaction at constant pressure, while the
bomb calorimeter measures the heat of a reaction at constant
volume. Our goal today is to introduce two important
thermodynamic quantities,  E, the internal energy and the energy associated with
a reaction at constant volume and
E, the internal energy and the energy associated with
a reaction at constant volume and  H, the enthalpy, the energy associated
with a reaction at constant pressure. Introducing and discussing
these two quantities is important to reaching our goal of
calculating heats of chemical reactions.
H, the enthalpy, the energy associated
with a reaction at constant pressure. Introducing and discussing
these two quantities is important to reaching our goal of
calculating heats of chemical reactions.
In our calorimetry calculations and in several of the problems in PS10, we
take advantage of the 1st Law of Thermodynamics, that energy is conserved. Energy
that was lost by the system, in an exothermic reaction, was absorbed by the
surroundings (the water and the calorimeter). Now we'll take a moment to apply
some of these concepts in the first law of thermodynamics. The first law states
that while energy can be converted from one form to another, it can not be created
or destroyed. When a chemical reaction occurs energy lost by the system must
be gained by the surroundings, and visa versa. This is also known as the law
of conservation of energy.
The energy we are discussing is the sum of all the kinetic
and potential energies of the systems component parts. This
includes the motion of the atoms, or molecules, electrons and
nuclei. This total energy is called the internal energy of the
system. We use the symbol, E, to represent the internal energy of
a system. The exact amount of the internal energy can not be
determined. However, we can measure the change in internal energy
of the system by measuring changes in temperature. For any
physical or chemical change the change in internal energy is
given as, (In chemical reactions the initial state is the
reactants and the final state is the products. )
 E
= Efinal - Einitial
E
= Efinal - Einitial  E = Eproducts - Ereactants
E = Eproducts - Ereactants
According to the first law
 Esystem
+
Esystem
+ Esurroundings
= 0
Esurroundings
= 0
or
 Esystem
= -
Esystem
= - Esurroundings
Esurroundings
An important point is the sign of  E. If
E. If  E is positive that means the Efinal is
greater than Einitial. If
E is positive that means the Efinal is
greater than Einitial. If  E is negative it is the reverse.
E is negative it is the reverse.
In a chemical equation we can locate the internal energy depending
on whether the reaction is endothermic or exothermic. For example, consider
the reaction between dihydrogen and dioxygen to produce water;
2H2(g) + O2(g) ---> 2H2O(g)
This reaction is exothermic so heat would be located in the
products;
2H2(g) + O2(g) --->
2H2O(g) + heat
We can draw a representation of this reaction. In the diagram
the internal energy of the reactants is greater than the internal
energy of the products. When the reaction proceeds from reactants
to products energy is released (we saw it). This is
characteristic of an exothermic reaction.
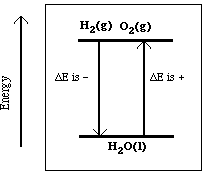
Another reaction, which we did in class, is described in the chemical equation;
Ba(OH)2.8H2O(s)
+ 2NH4Cl(s) ---> BaCl2(aq) +
2NH3(g) + 10H2O(l)
Recall this reaction was endothermic, so heat is located on
the reactants side of the reaction.
heat + Ba(OH)2.8H2O(s)
+ 2NH4Cl(s) ---> BaCl2(aq) +
2NH3(g) + 10H2O(l)
In the diagram below the reactants are lower in energy
compared to the products. As the reaction proceeds energy must be
added. Notice the separation between reactants and products is
different in the two diagrams. The separation is directly related
to the magnitude of the change in the internal energy.
By definition if the system givens off heat, exothermic,  E is -. If heat is
absorbed by the system
E is -. If heat is
absorbed by the system  E is +.
E is +.
According to the first law of thermodynamics a particular
chemical system can transfer energy with it surroundings in the
form of heat or work. Energy is transferred by adding or removing
heat, or by doing or having work done on it. So when a system
undergoes a chemical or physical change, the change in internal
energy is given by the heat added to the system plus the work
done on the system. The equation is,
 E
= q + w
E
= q + w
There are many kinds of work, but in the chemical systems we
are interested mechanical work done by expanding gases is the way
reactions can do work. (Recall that expanding gases, from the
combustion of gasoline, do work on the piston which eventually
turns the wheels.)
w is P V for expanding gases
V for expanding gases
When a reaction system is open to the atmosphere the reaction
does work on the surroundings, this is expressed;
as w = -P V
V
Work done by the system on the surrounding has a negative
sign by convention. Reactions performed in the open atmosphere do
work on the atmosphere, but this type of work by a system does
nothing in a practical sense.
If in a chemical reaction the work done is accomplished by expansion
then we can substitute for w in the equation;  E
= q + w
E
= q + w
yields,
 E
= q - P
E
= q - P V
V
so the change in internal energy for a system can be
determined by measuring the heat transferred in the reaction and
calculating P V.
V.
If the chemical reaction occurs in a device which the volume
of the reaction is held constant, then  V = 0 and the change in internal energy is
equal to heat transferred in the reaction. Recall for the
reaction done at constant volume in the 'bomb' calorimeter we
would expect
V = 0 and the change in internal energy is
equal to heat transferred in the reaction. Recall for the
reaction done at constant volume in the 'bomb' calorimeter we
would expect  V to
equal 0. So under these conditions
V to
equal 0. So under these conditions
qv (constant volume) =  E
E
So any heat gained or lost is equal to the change in internal
energy.
If we perform a chemical reaction at constant volume and can
measure the heat transferred in the reaction we can calculate  E for the reaction.
E for the reaction.
What happens if we perform the reaction at constant pressure
rather than constant volume? This is the case of most chemical
reactions performed in the laboratory. In the case of a reaction
open to the atmosphere the pressure is constant, not volume. We
begin with our original statement of the first law,
 E
= q -P
E
= q -P V
V
and define q as qp the heat transferred at
constant pressure.
 E
= qp - P
E
= qp - P V
V
or
qp (constant pressure) =  E + P
E + P V
V
qp the heat evolved or absorbed at constant
pressure and is very important for most chemical system. The
reactions we study in the laboratory are at constant pressure. qp
is called enthalpy and written as  H (enthalpy).
H (enthalpy).
 H
= qp =
H
= qp =  E
+ P
E
+ P V
V
The heat flow at constant pressure, qp, can be
measured using a coffee-cup calorimeter. The reaction is
performed in the water in the calorimeter and heat is absorbed or
given up by the water and the calorimeter.
When a reaction is run at constant pressure open to the atmosphere it is difficult
to calculate P V. Recall that according to the ideal gas law (PV = nRT)
we can substitute
V. Recall that according to the ideal gas law (PV = nRT)
we can substitute  nRT for P
nRT for P V.
V.
 nRT is calculable from the information
provided.
nRT is calculable from the information
provided.  n is equal nproducts gases - nreactants gases,
R has the value of 8.314 J.mol-1.K-1 and T
is the temperature in Kelvins.
n is equal nproducts gases - nreactants gases,
R has the value of 8.314 J.mol-1.K-1 and T
is the temperature in Kelvins.
If the enthalpy of the products is greater than the enthalpy
of the reactants  H
is positive, the reaction is endothermic. On the other hand when
the enthalpy of the reactants is greater than the enthalpy of the
products
H
is positive, the reaction is endothermic. On the other hand when
the enthalpy of the reactants is greater than the enthalpy of the
products  H is
negative, the reaction is exothermic. For the reactions I
demonstrated earlier,
H is
negative, the reaction is exothermic. For the reactions I
demonstrated earlier,  H = - 286 kJ (1/2H2 + O2), and
+167 kJ (Ba(OH)2 + NH4SCN).
H = - 286 kJ (1/2H2 + O2), and
+167 kJ (Ba(OH)2 + NH4SCN).
We can write the chemical equation as,
1/2H2(g) + O2(g) --->
H2O(g) + 286 kJ
or
1/2H2(g) + O2(g) --->
H2O(g)  H = -286 kJ
H = -286 kJ
167 kJ + Ba(OH)2.8H2O(s)
+ 2NH4Cl(s) ---> BaCl2(aq) +
2NH3(g) + 10H2O(l)
or
Ba(OH)2.8H2O(s)
+ 2NH4Cl(s) ---> BaCl2(aq) +
2NH3(g) + 10H2O(l)  H = 167 kJ
H = 167 kJ
We need to consider four important properties when using
enthalpy in chemical reactions.
Enthalpy is an extensive property
The magnitude of  H depends directly on the
amount of reactant consumed. In the reaction
between hydrogen and oxygen,
H depends directly on the
amount of reactant consumed. In the reaction
between hydrogen and oxygen,
1/2H2(g) + O2(g)
---> H2O(g)  H = -286
kJ
H = -286
kJ
The equation says that when 1 mol of hydrogen
is combined with 0.5 moles of oxygen, 1 mol of
water is formed and 286 kJ of energy is
liberated. If 2 moles of hydrogen are combined
with 1 mol of oxygen to form 2 moles of water the
amount of energy produced is twice as much, or
572 kJ.
The enthalpy change for a reaction is equal in
magnitude and opposite in sign to  H for the reverse reaction.
H for the reverse reaction.
For the reaction, 1/2H2(g) + O2(g)
---> H2O(g)  H = -286
kJ
H = -286
kJ
if the reaction is reversed,
H2O(g)
---> 1/2H2(g) + O2(g)  H = +286
kJ
H = +286
kJ
The enthalpy of the reaction depends on the state of
the reactants and products.
For the reaction, 1/2H2(g) + O2(g)
---> H2O(g)  H = -286
kJ
H = -286
kJ
however, if the state of the water formed is
gaseous instead of liquid  H = - 242 kJ. Changing the
phase of a substance involves a change in the
enthalpy. In this example, an additional amount
of energy is required to change water in the
liquid phase to water in the vapor phase (44 kJ).
H = - 242 kJ. Changing the
phase of a substance involves a change in the
enthalpy. In this example, an additional amount
of energy is required to change water in the
liquid phase to water in the vapor phase (44 kJ).
For meaningful comparisons of heats of
reaction chemists agree to specify the physical
state of all substances in the reaction and the
temperature at which the reaction was measured.
If the reactants and products are at 25 C and 1
atmosphere they are referred to standard state
enthalpies and the enthalpy of the reaction is
written as  H. Unless otherwise indicated all the
reactions will have the reactants and products in
their standard states.
H. Unless otherwise indicated all the
reactions will have the reactants and products in
their standard states.
Hess' law says, if a reaction is carried out in a
series of steps,  H for the reaction will equal the sum of the
enthalpy changes for each step.
H for the reaction will equal the sum of the
enthalpy changes for each step.
-
In order to demonstrate this property of enthalpy I need to define
state function. Both enthalpy,  H and internal energy,
H and internal energy,  E are examples of state
functions, in that they are properties of a system which are determined
by specifying its condition or its state. The value of a state function
does not depend on the history of the sample, only on its current condition.
A state function does not depend on the path used to get the system
to that state. Mathematically this statement can be expressed as,
E are examples of state
functions, in that they are properties of a system which are determined
by specifying its condition or its state. The value of a state function
does not depend on the history of the sample, only on its current condition.
A state function does not depend on the path used to get the system
to that state. Mathematically this statement can be expressed as,
 X = Xfinal
- Xinitial
X = Xfinal
- Xinitial
For X to be a state function  X is the same independent
of how the system is changed from the initial state to the final state.
Let's consider an analogy to this in the form of a trip. If we travel
by driving from Stillwater, Oklahoma (elevation 913 ft, latitude 97
degree 0 ', longitude 36 degree 0 ') to Bellingham, Washington (elevation
0 ft, latitude 122 degree 30 ', longitude 48 degree 50 ') there are
a large number of routes that could be selected. Properties of the different
routes that are not state functions are, distance, cost, tire wear and
time. Properties that are state functions are the difference in altitude
between Stillwater and Bellingham, the difference in longitude or latitude.
These properties are all state functions. They only depend on the initial
and final point of the trip, not on what happens between the initial
point and the final point of the trip.
X is the same independent
of how the system is changed from the initial state to the final state.
Let's consider an analogy to this in the form of a trip. If we travel
by driving from Stillwater, Oklahoma (elevation 913 ft, latitude 97
degree 0 ', longitude 36 degree 0 ') to Bellingham, Washington (elevation
0 ft, latitude 122 degree 30 ', longitude 48 degree 50 ') there are
a large number of routes that could be selected. Properties of the different
routes that are not state functions are, distance, cost, tire wear and
time. Properties that are state functions are the difference in altitude
between Stillwater and Bellingham, the difference in longitude or latitude.
These properties are all state functions. They only depend on the initial
and final point of the trip, not on what happens between the initial
point and the final point of the trip.
Returning to the fourth property, enthalpy is a state function. It
does not depend on how we reached a particular state of the system,
only on the initial and final states.
Hess's Law - in going from a particular set of reactants to a particular
set of products, the enthalpy change is the same whether the reaction
takes place in one step or in a series of steps.  H for the overall reaction
will be equal to the sum of the enthalpy changes of each step.
H for the overall reaction
will be equal to the sum of the enthalpy changes of each step.
Lets consider several sample problems to demonstrate how we
apply Hess' Law and several other properties of enthalpy.
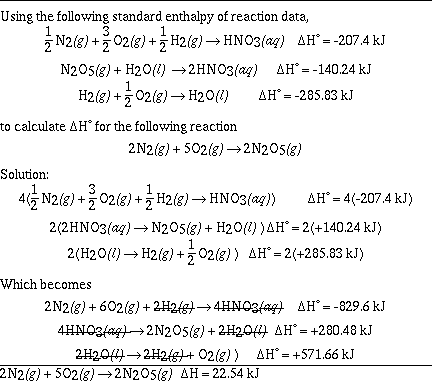
Sample Problem #1 (Hess' Law);

Sample Problem #2 (Hess' Law);

Sample Problem #3 (Hess' Law);
As you might imagine it might be possible to experimentally
collect  H data for a
number of reactions, tabulate these reactions and their
corresponding enthalpy, and then use those reactions and Hess'
Law to calculate the
H data for a
number of reactions, tabulate these reactions and their
corresponding enthalpy, and then use those reactions and Hess'
Law to calculate the  H
for other reactions. But to be as efficient as possible a
particular type of reaction is selected. The type of reaction is
called a formation reaction. A formation reaction is defined 1
mole of a compound in its standard state is formed from its
elements in their standard states. I have already shown how the
magnitude of the enthalpy depends on the temperature, pressure,
state of the reactants and products, so a standard state is
defined as the state of a substance at 25 C and 1 atmosphere
pressure. The heat associated with this reaction, at constant
pressure, is the standard heat of formation,
H
for other reactions. But to be as efficient as possible a
particular type of reaction is selected. The type of reaction is
called a formation reaction. A formation reaction is defined 1
mole of a compound in its standard state is formed from its
elements in their standard states. I have already shown how the
magnitude of the enthalpy depends on the temperature, pressure,
state of the reactants and products, so a standard state is
defined as the state of a substance at 25 C and 1 atmosphere
pressure. The heat associated with this reaction, at constant
pressure, is the standard heat of formation,  Hf. We have
already encountered three examples of standard heat of
formations,
Hf. We have
already encountered three examples of standard heat of
formations,  Hf
for CO(g), CO2(g) and H2O(l).
Hf
for CO(g), CO2(g) and H2O(l).
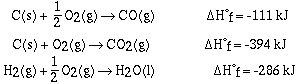
Recall that the enthalpy change for a reaction is given as;
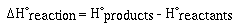
For the formation of CO2 we would write,
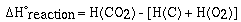
We are only able to determine the change,  H, in enthalpy for a
chemical reaction. We do not know the enthalpy for a substance.
By convention the enthalpy of an element in its standard state is
zero. Therefore,
H, in enthalpy for a
chemical reaction. We do not know the enthalpy for a substance.
By convention the enthalpy of an element in its standard state is
zero. Therefore,
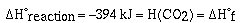
This list of heats (enthalpies) of formation can be used to
calculate heats of reactions. To do this we use the equation,
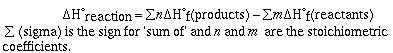
Lets do some sample problems;
Sample Problem #1 (Heat of Formation);
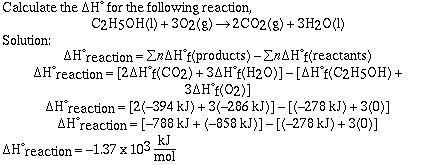
Sample Problem #2;

Sample Problem #3 (Heat of Formation);