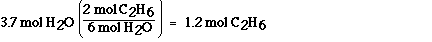According to the chemical equation
2C2H6(g) + 7O2(g) --->
4CO2(s) + 6H2O(g)
c) how many mol of ethane, assuming excess dioxygen, are
required to form 3.7 mol of water?
Answer
The approach is nearly identical to that used in part a). We
must use the stoichiometric ratio between water and ethane to
answer the question. This ratio is obtained from the balanced
chemical equation. According to the balanced chemical equation
six mol of water are formed when two mol of ethane, C2H6,
react. Water and ethane always combine in this ratio according to
this equation. We can use this relationship as a unit conversion
factor to convert between mol of water and mol of ethane.