In the reaction
2C2H6(g) + 7O2(g)
---> 4CO2(g) + 6H2O(g)
Determine the mol of carbon dioxide formed when 2.00
mol of ethane are reacted with 10.0 mol of oxygen.
To solve this limiting reagent problem I recommend
using a table approach which I have developed over the years. To solve
this problem we prepare a table which looks like the following;
(mol reactant A)o
|
(mol reactant B)required
|
(mol reactant B)o
|
Conclusion
|
|
|
|
|
To fill out the table we arbitrarily select one of
the reactant (it does not make a difference which one) as reactant
A. Once reactant A is selected reactant B is defined. So if we select
reactant A as C2H6, the reactant B must be O2.
So the table would look like;
(mol reactant C2H6)o
|
(mol reactant O2)required
|
(mol reactant O2)o
|
Conclusion
|
|
|
|
|
Now we can add the mol of each reactant initially and
the mol of O2 required. according to the statement of the
problem we are given 2.00 mol of C2H6 and 10.0
mol of O2 initially.
(mol reactant C2H6)o
|
(mol reactant O2)required
|
(mol reactant O2)o
|
Conclusion
|
2.00
|
|
10.0
|
|
To determine the mol of O2 required, we
must answer the question how many mol of O2 are required
to react with 2.00 mol of C2H6. So the following
calculation;
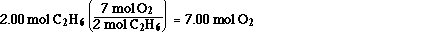
This calcualtion says 7.00 mol of O2 are
required to completely react with 2.00 mol of C2H6.
So the table would now look like,
(mol reactant C2H6)o
|
(mol reactant O2)required
|
(mol reactant O2)o
|
Conclusion
|
2.00
|
7.00
|
10.0
|
|
To determine the limiting reagent we compare the mol
of O2 required to mol of O2 initially. In this
case 7.00 mol O2 are required and we have 10.0 mol O2
initially. So we have initially more mols of O2 than are
required. So O2 is in excess and C2H6
is limiting.
(mol reactant C2H6)o
|
(mol reactant O2)required
|
(mol reactant O2)o
|
Conclusion
|
2.00
|
7.00
|
10.0
|
O2 is excess
C2H6 is limiting
|
Now we use the limiting reagent to calculate the amount
of CO2 formed.

|