Perform the following conversion and report the answer to the
correct number of significant figures.
c) The estimated water content in moon rock is 0.100 % by
mass. Determine the mass of moon rock needed to extract 1.00
gallon of water.
Answer
This is another density problem, because we are given a
volume of water and told to determine the a mass. This problem is
a little more complicated. We know how many grams of water are in
100. grams of moon rock. If the moon rock is 0.100% water by
mass, then if we have 100 grams we know there is 0.1 grams of
water. So how many grams of water are in a gallon of water is the
next question. To determine that we'll begin with a gallon of
water and convert that to grams of water in the following way.
Oh, remember the density of water is 1.0 g mL-1.

So now we know how many mLs there are in a
gallon. We can now use density to find the grams,
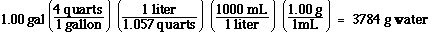
Using the relationship between grams of water
and grams of moon rock,
0.1 g water = 100 g moon rock
we determine the mass of the moon rock which
contains 3784 g of water.
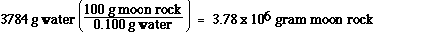
![]()
![]()
![]()