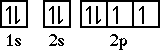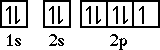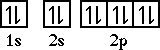Chapter 11: Introduction to Modern Atomic TheoryThe next element is oxygen. Oxygen has an electron configuration of 1s22s22p4. With nitrogen all three of the orbitals in the 2p sublevel are half-filled. So with oxygen the fourth electron must go into one of the half-filled orbitals. The orbital diagram for oxygen is,
After oxygen is fluorine. Fluorine has an electron configuration of 1s22s22p5. The orbital diagram for fluorine is,
Notice that fluorine is one electron short of a filled shell. Fluorine (and all the halogens/Group VIIA elements) will gain one electron when it combines with other elements.Next comes neon, a noble gas. Neon has an electron configuration of 1s22s22p6. The orbital diagram for neon is,
With neon the second level is filled. Neon is unreactive, no compounds containing neon are known.After neon come sodium. Adding one more electron requires moving to the third level. The third level has three sublevels; 3s, 3p and 3d. As was the case in the second level the 3s sublevel falls in energy relative to the 3p and 3d sublevels.So the electron configuration for sodium is 1s22s22p63s1. Notice sodium has one electron in its outer most energy level.*NOTE: This file will load very slowly if you are trying to download on a 56K modem. View this movie using QuickTime 4 on the campus network. |