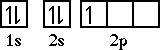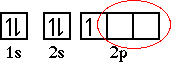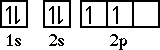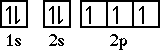Chapter 11: Introduction to Modern Atomic TheoryThe next element is carbon. Carbon has an electron configuration of 1s22s22p2. We must be careful doing the orbital diagram for carbon. Lets look at the orbital diagram for carbon before we place the sixth electron.
Adding the second electron into the 2p sublevel we have to careful. We can not place the second electron into the orbital that already has one electron. Recall I mentioned earlier it takes some energy to have two electrons in the same orbital, due to the replusion experienced by the like charges. So the second electron must go into an orbital that does not already contain an electron. There are two such orbitals,
Either one will work. So the correct orbital diagram for carbon becomes,
The next element is nitrogen. Nitrogen has an electron configuration of 1s22s22p3. The orbital diagram for nitrogen is,
Nitrogen has three unpaired electrons. The orbital diagram clearly indicates the number of unpaired electrons for an atom. The electron configuration is not as revealing.*NOTE: This file will load very slowly if you are trying to download on a 56K modem. View this movie using QuickTime 4 on the campus network. |