|
Chapter 11: Introduction to Modern Atomic Theory
The next element is beryllium which has four
electrons. Where do we place the fourth electron? The 2s
and 2p sublevels are very close. Which sublevel do we
place the fourth electron? We place it into the 2s
sublevel.
Beryllium has an electron configuration of 1s22s2.
The orbital diagram looks like,
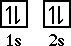
Does beryllium, with an electron configuration of 1s22s2,
have a filled shell? No.
The reason is that all of the orbitals in the second
level are not yet full. The 2p sublevel is completely
empty.
After beryllium comes boron with five electrons.
The electron configuration for boron is
1s22s22p1. The orbital
diagram for boron is...well let's look at the orbital
diagram before we place the fifth electron.

The three orbitals in the 2p sublevel
are all the same energy, they are degenerate in energy,
so it really makes no difference whether we write and
orbital diagram as,
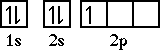 or or  or or 
Notice in the electron configuration for
boron (1s22s22p1) we do
not worry about which orbital the electron is located, it
is only in the orbital diagram that we must be concerned
about such details.
*NOTE: This file will load very slowly if you are
trying to download on a 56K modem. View this movie using
QuickTime 4 on the campus network.
|

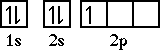 or
or  or
or 