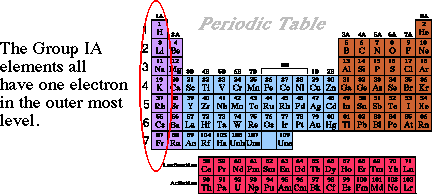
Chapter 11: Introduction to Modern Atomic TheoryHow many electrons does hydrogen have in its outer most energy level?The outer most energy level is defined as the highest level that contains an electron. Since the only electron in the hydrogen atom is in the n = 1 level, that is the highest energy level. There is only one electron in this level, so the answer to the question is...one electron.How many electrons in the outer most energy level in lithium? In the case of lithium the highest energy level is the n= 2 level and there is one electron in the second level.If we continue down the column of alkali metals in the periodic table looking at sodium, potassium rubidium, etc. They ALL have one electron in their outer most energy level!
Helium has two electrons in 1s orbital. When the second electron goes into the 1s orbital for helium that orbital is filled. Also the 1s subshell is filled and the n = 1 level is filled. The first level is filled, we can not put anymore electrons into this shell. When an atom has a filled shell there is a special stability.Helium is a noble gas, it does not react with any other substance. When an atom has a filled shell it does not want to gain or lose electrons! A filled shell lends special stability.Neon also has a filled shell and is especially stable.Other atoms will gain or lose electrons to achieve the filled shell arrangement.Lithium will lose one electron to achieve the same electron configuration (filled shell) that helium has. A neutral lithium atom has three electrons, one of which is in the second level. By loosing the outer most electron it is like helium. Lithium is VERY reactive because it wants to loose that outer most electron.Sodium likes to lose one electron also. A neutral sodium atom has 11 electrons, when it does it loses the outer most electron is has the same electron configuration as neon. Sodium is reacts to lose one electron in all of its chemistry.Potassium, rubidium, cesium all lose one electron.Would you like to try a few questions to see if this section is clear?*NOTE: This file will load very slowly if you are trying to download on a 56K modem. View this movie using QuickTime 4 on the campus network. |