|
Chapter 11: Introduction to Modern Atomic Theory
When we go to helium where do we place the second
electron?
The rule is we write the electron configuration for
the atom in its lowest energy state. So we must place the
second electron in the orbital with the lowest energy. An
orbital in the second level (n = 2) is higher in energy
compared to placing the second electron into the same
orbital that we placed the first electron.
Helium has an electron configuration of 1s2.
The orbital diagram looks like,
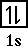
It does take some energy to place two electrons into
the same orbital. Recall that electrons are negatively
charged. Two electrons in the same orbital repell each
other, so it takes some energy to place the second
electron into an already half-filled orbital.
*NOTE: This file will load very slowly if you are
trying to download on a 56K modem. View this movie using
QuickTime 4 on the campus network.
|