|
Chapter 11: Introduction to Modern Atomic Theory
In our Quantum Mechanical model the energy levels
also have sublevels and orbitals.
In the first level there is one sublevel which we can
the s sublevel. In the second level there are two
sublevels, the s sublevel and the p
sublevel. In the third level there are three
sublevels....etc.
In the Periodic Table the sublevels are evident by
the groupings of the columns. The s sublevel is
located on the left side of the periodic table, the p
sublevel on the right. The transition metals are located
in the d sublevel. Down the side are the period
numbers those numbers correspond to the level the
electron can be located.
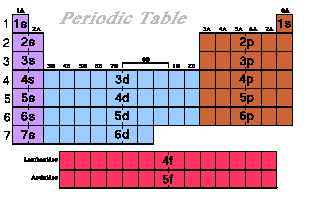
Recall the s sublevel has one orbital, a p
sublevel has three orbitals, and a d sublevel
has five orbitals.
*NOTE: This file will load very slowly if you are
trying to download on a 56K modem. View this movie using
QuickTime 4 on the campus network.
|
