Calculate the temperature change when 1575 J of heat are
removed from an 85.0 gram sample of ethyl alcohol originally at
23.5 °C.
Answer.
We can obtain the specific heat of ethyl alcohol from the
table in our lecture notes. It is 2.45 J g-1 °C-1.
Using the relationship,

we need to rearrange to solve for temperature,

Substituting,
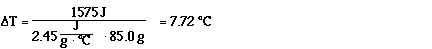
So the temperature change is 7.72 °C.
Now can you calculate the final temperature of the sample of
ethyl alcohol?
This is a little tricky, what we have to what out for is that
the heat is REMOVED! So the final temperature is,
Tfinal = 23.5 °C - 7.72 °C = 15.8
°C