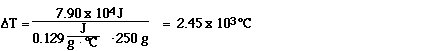Calculate the change in temperature when 7.90 x 104
J (the same amount of heat to boil a cup of water) of heat are
added to a 250 gram sample of gold.
Answer.
We can obtain the specific heat of gold from the table in our
lecture notes. It is 0.129 J g-1 °C-1.
Using the relationship,

we need to rearrange to solve for temperature,

Substituting,
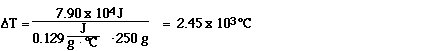
Now this is interesting. The same amount of heat required to
boil 250 grams of water translates to a temperature change of
2450 °C. The boiling point of gold is 1064 ÐC. So adding this
amount of heat will change the 250 gram sample of gold into
vapor!